As the world deals with the COVID-19 pandemic, vaccination remains vital to successfully end this crisis. However, COVID-19-vaccine-induced immediate hypersensitivity reactions presenting with potentially life-threatening systemic anaphylactic reactions are one of the reasons for vaccine hesitancy. SRecent studies have suggested that different mechanisms, including IgE-mediated and non-IgE-mediated mast cell activation, may be involved in immediate hypersensitivity. The main culprits triggering hypersensitivity reactions have been suggested to be the excipients of vaccines, including polyethylene glycol and polysorbate 80. Patients with a history of allergic reactions to drugs, foods, or other vaccines may have an increased risk of hypersensitivity reactions to COVID-19 vaccines. Various strategies have been suggested to prevent hypersensitivity reactions, including performing skin tests or in vitro tests before vaccination, administering different vaccines for the primary and following boosters, changing the fractionated doses, or pretreating the anti-IgE antibody.
- COVID-19 vaccines
- IgE-mediated pathway
- immediate hypersensitivity reactions
- skin test
1. Introduction
2. Clinical Phenotypes of Vaccine-Induced Immediate Hypersensitivity Reactions
Although vaccination has dramatically improved the control of COVID-19 transmission [11], vaccination hesitancy remains a significant issue owing to adverse reactions, particularly unpredictable hypersensitivity reactions [12][13][12,13]. Most hypersensitivity reactions to vaccines occur immediately and abruptly within minutes to hours after administration [14][15][16][14,15,16]. The clinical manifestations may range from mild cutaneous eruptions, such as urticaria or angioedema, to life-threatening systemic anaphylaxis [17]. Urticaria is characterized by transient wheal formation and may produce an itching or burning sensation. Angioedema is characterized by painful swelling in the deep dermis and subcutis layers of the skin. Both presentations are part of a spectrum of systemic symptoms, including anaphylaxis [18]. Anaphylaxis is rare but frequently leads to death [19][20][19,20]. Most immediate hypersensitivity reactions have occurred after administrating the first dose. However, reactions after the second dose of the COVID-19 vaccine have also been reported [21]. Approximately 86% of anaphylaxis cases induced by COVID-19 vaccines occur within 30 min of inoculation. On the contrary, the onset of other symptoms, such as urticaria, often happens within 3–8 days of the first dose and 2–5 days after the second dose [21][22][23][21,22,23]. Many vaccine-induced hypersensitivity reactions could not be confirmed and have been attributed post factum to alternative diagnoses, such as vasovagal syncope, vocal cord dysfunction, exacerbation of existing chronic spontaneous urticaria, and anxiety. Using an updated global standard for case definitions and guidelines for hypersensitivity reactions following vaccinations may help with clinical differential diagnosis and management [24][25][24,25].3. Epidemiology of Immediate Hypersensitivity Induced by Vaccines
Vaccine-induced anaphylaxis cases are estimated to occur in approximately 1 case per 15 million to 2 cases per million individuals [14]. Micheletti F. et al. reported that the risk of anaphylaxis after vaccination in children and adults was estimated to be 1.31 (95% confidence interval [CI], 0.90~1.84) per million doses before the COVID-19 pandemic [26]. The authors identified 33 confirmed vaccine-triggered anaphylaxis cases in the study after 25,173,965 vaccine doses [26]. Among the patients with vaccine-induced immediate hypersensitivity reactions, approximately 66% had urticaria, and 10% had angioedema [27]. For COVID-19 vaccines, cutaneous reactions were reported by 1.9% of individuals after receiving the first dose of an mRNA COVID-19 vaccine. Approximately 2.3% of those who had no adverse events following the first dose developed hypersensitivity reactions after receiving the second dose [28]. Based on a U.S. study, cutaneous reactions induced by the mRNA COVID-19 vaccines were more common in women than in men (85% vs. 15%, p < 0.001) [28]. Furthermore, the estimated incidence rates for anaphylaxis in the U.S. were 11.1 cases per million doses administered with the BNT162b2 (Pfizer-BioNTech) vaccine and 2.5 cases per million doses administered with the mRNA-1273 (Moderna) vaccine [16][29][30][31][16,29,30,31]. The vaccine adverse event reporting system (VAERS) [32] showed that there were 1592 urticaria cases among 15703 (10.13%) cases with adverse reactions, 32 (4.92%) out of 650 adverse event cases of angioedema, and 66 (3.54%) out of 1867 adverse event cases of anaphylaxis from 2020 to January 2022 attributed to COVID-19 vaccines. A recent meta-analysis study suggested that the estimated incidence of COVID-19-vaccine-induced anaphylaxis ranged from 2.5 to 7067 per one million individuals receiving mRNA COVID-19 vaccines, with an overall pooled prevalence estimate of 5.58 (95% CI, 3.04–8.12; I2 = 76.32%, p < 0.01) [21]. In contrast, the incidences of nonanaphylactic reactions to mRNA COVID-19 vaccines ranged from 10.6 to 472,973 per one million, with an overall pooled prevalence estimate of 89.53 (95% CI, 11.87–190.94; I2 = 97.08%, p < 0.01) [21]. Chu, DK. et al. performed a meta-analysis of 22 studies, including 1366 patients, and found a low incidence (0.16%) of immediate severe allergic reactions associated with the second dose of the mRNA COVID-19 vaccine among individuals who had an allergic history of their first dose [33]. In a separate study, the incidence rates of anaphylaxis were lower for the viral COVID-19 vaccine (odds ratio [OR], 0.47; 95% CI, 0.33–0.68) and the inactivated COVID-19 (OR, 0.31; 95% CI, 0.18–0.53) vaccine [34]. Different setups of studies may observe different incidence rates. Table 1 lists the incidence rates of anaphylactic and nonanaphylactic hypersensitivity reactions to COVID-19 vaccines.| Type of Reaction | Number of Participants | Number of Anaphylactic Reactions | Type of Vaccine | Incidence of Reactions (per One Million) | Reference |
|---|---|---|---|---|---|
| anaphylactic | |||||
| 890,604 | 15 | mRNA-1273; BNT162b2 | 17 | [35] | |
| 4,041,396 | 10 | mRNA-1273 | 37.1 | [29] | |
| 1,893,360 | 21 | BNT162b2 | 11 | [36] | |
| 1116 | 1 | BNT162b2; mRNA-1273 | 890 | [37] | |
| 283 | 5 | mRNA-1273 and AZD1222 | 17,668 | [38] | |
| nonanaphylactic | |||||
| 277 | 14 | BNT162b2 | 50,540 | [39] | |
| 5589 | 1391 | AZD1222 (Astra Zeneca) |
248,880 | [39] | |
| 5574 | 6 | BNT162b2 | 1070 | [40] | |
| 3170 | 11 | BNT162b2 | 3470 | * [41] | |
| 1,893,360 | 83 | BNT162b2 | 43.8 | * [36] | |
| 877 | 10 | BNT162b2 | 11,400 | [42] | |
| 1116 | 7 | BNT162b2; mRNA-1273 | 6270 | [37] | |
| 74 | 35 | BNT162b2 | 472,973 | [23] |
4. Causality of Vaccine-Induced Immediate Hypersensitivity Reactions
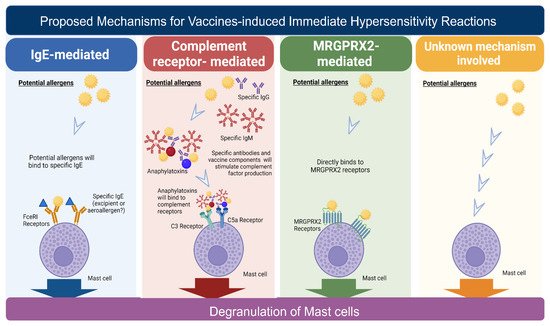
Vaccine excipients and active components could cause allergens to elicit hypersensitivity reactions. These antigen components, such as toxoids or constituents of pneumococcal vaccines, cause symptoms ranging from urticaria to anaphylaxis. Hypersensitivity reactions may be induced when patients receive the first or the second dose of a vaccine [48][49][48,49]. Vaccine excipients are known to be ingredients other than the active components of vaccines. These are inactive ingredients that stabilize or preserve the viability of the vaccines and maintain their bioavailability. Egg and ovalbumin (a residual component of egg processing) are considered the most frequent food allergies in children and the most suspected culprits for allergies induced by the administration of traditional vaccines [49][50][51][52][49,50,51,52]. Gelatin is another culprit excipient for vaccine-induced immediate hypersensitivity reactions [51][52][53][51,52,53]. Vaccine adjuvants are also possible allergens [54]. Aluminum hydroxide and aluminum phosphate are adjuvants that are more commonly found in vaccines but are not in the COVID-19 vaccine. Although rare, they are commonly associated with delayed-type hypersensitivity reactions. Aluminum can also induce immediate-type hypersensitivity by stimulating mast cells and other immune cells [49][55][49,55]. Another vaccine adjuvant, AS03, is a squalene derivative that is incorporated into influenza vaccines. Epidemiological studies in Canada have shown an approximately 20-fold increase in the incidence of immediate hypersensitivity using AS03-adjuvanted vaccines compared with non-AS03 vaccines. The immune mechanism underlying vaccine-adjuvant-induced immediate hypersensitivity reactions remains unclear [49][54][56][57][49,54,56,57].5. Proposed Immune Mechanisms for Vaccine-Induced Immediate Hypersensitivity Reactions
According to cellular and molecular features defined by Gell and Coomb, there are four types of hypersensitivity reactions: I, II, III, and IV [58]. Type I hypersensitivity reactions involve IgE-mediated immune responses and occur rapidly after exposure to allergens. Type II hypersensitivity is mediated by IgG or IgM antibodies, and type III hypersensitivity involves the immune complexes. Type IV hypersensitivity is mediated by T lymphocytes, also known as delayed-type reactions. Mast cells are considered the most critical immune cells responsible for immediate hypersensitivity reactions, as they secrete various inflammatory cytokines and induce various systemic immune responses [52]. There are four proposed mechanisms for immediate hypersensitivity reactions, including (1) immunoglobulin E (IgE)-mediated, (2) complement-receptor-mediated, (3) MRGPRX2 (Mas-related G-protein coupled receptor member X2)-mediated mast cell direct activation, and (4) an unknown mechanism (Figure 1).