Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianluca Natrella | -- | 1819 | 2022-05-30 10:38:51 | | | |
| 2 | Vivi Li | -13 word(s) | 1806 | 2022-05-31 03:23:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Natrella, G.; De Falco, M.; Capocelli, M.; , .; Sołtysik, M.; Wawrzyńczak, D.; Majchrzak-Kucęba, I. DME Synthesis from CO2 and Renewable Hydrogen. Encyclopedia. Available online: https://encyclopedia.pub/entry/23545 (accessed on 13 January 2026).
Natrella G, De Falco M, Capocelli M, , Sołtysik M, Wawrzyńczak D, et al. DME Synthesis from CO2 and Renewable Hydrogen. Encyclopedia. Available at: https://encyclopedia.pub/entry/23545. Accessed January 13, 2026.
Natrella, Gianluca, Marcello De Falco, Mauro Capocelli, , Marcelina Sołtysik, Dariusz Wawrzyńczak, Izabela Majchrzak-Kucęba. "DME Synthesis from CO2 and Renewable Hydrogen" Encyclopedia, https://encyclopedia.pub/entry/23545 (accessed January 13, 2026).
Natrella, G., De Falco, M., Capocelli, M., , ., Sołtysik, M., Wawrzyńczak, D., & Majchrzak-Kucęba, I. (2022, May 30). DME Synthesis from CO2 and Renewable Hydrogen. In Encyclopedia. https://encyclopedia.pub/entry/23545
Natrella, Gianluca, et al. "DME Synthesis from CO2 and Renewable Hydrogen." Encyclopedia. Web. 30 May, 2022.
Copy Citation
Carbon Capture and Utilization (CCU) is a viable solution to valorise the CO2 captured from industrial plants’ flue gas, thus avoiding emitting it and synthesizing products with high added value. The first step in a CCU process is the capturing of CO2 through well-known technologies, such as oxyfuel combustion, pre-combustion or post-combustion, or as a direct-air capture process. Post-combustion carbon capture can be achieved by physical or chemical separation methods, such as membranes, adsorption, absorption and cryogenic processes. After having captured and concentrated the CO2, it can be fed to a chemical reactor for its conversion into products, such as syngas, urea, methane, ethanol, formic acid, etc.
carbon capture and utilization
methanol and DME production
exergy analysis
1. Introduction
Climate change mitigation is a worldwide effort that involves all countries around the world. Among all problems, greenhouse gas (GHG) emissions have a significant impact on the environment. Regarding this matter, the Intergovernmental Panel on Climate Change (IPCC) and the United Nations Climate Change Conference have established the needs of reducing CO2 emissions (recognized as the gas mainly responsible for climate change) and mitigating the global average temperature increase. Targets were set up, proposals to reach them were provided, and some technologies were identified as a solution in counteracting this issue.
The extensive concentration of carbon dioxide in the atmosphere is a threat to environmental safety, contributing to the greenhouse effect, but CO2 is a source of carbon for plants and can also be used as a reactant in chemical reactions [1][2][3]. This concept has led to the development of Carbon Capture and Utilization (CCU) technologies, which are perceived as a more justified and socially acceptable technology for CO2 management than Carbon Capture and Storage (CCS). However, even if they can be considered a feasible solution, their cost is still an issue. Hasan et al. proposed a national Carbon Capture Utilization and Storage (CCUS) supply chain network for a U.S. case study in their multiscale framework analysis [4]. With the proposed solution focused on profit rather than maximizing CO2 utilization, average profits between $0.3 and $17.6 per ton of CO2 were achieved (depending on the weighted average total costs of capturing and utilizing a ton of CO2).
There are plenty of ways to apply CCU technology wherever there is a CO2-emitting source, e.g., energy-intensive industry branches, such as energy, petrochemical and cement or iron and steel production. Additionally, CCU reactions can be supported with green technologies, such as renewable energy sources (RES).
The first step in a CCU process is the capturing of CO2 through well-known technologies, such as oxyfuel combustion, pre-combustion or post-combustion, or as a direct-air capture process [5][6]. Post-combustion carbon capture can be achieved by physical or chemical separation methods, such as membranes, adsorption, absorption and cryogenic processes. Many of these technologies are already applied in industry [7]. Pre-combustion processes capture CO2 prior to the combustion reaction and it can be achieved with the coal gasification process or with oil or gas fuel-reforming processes [8].
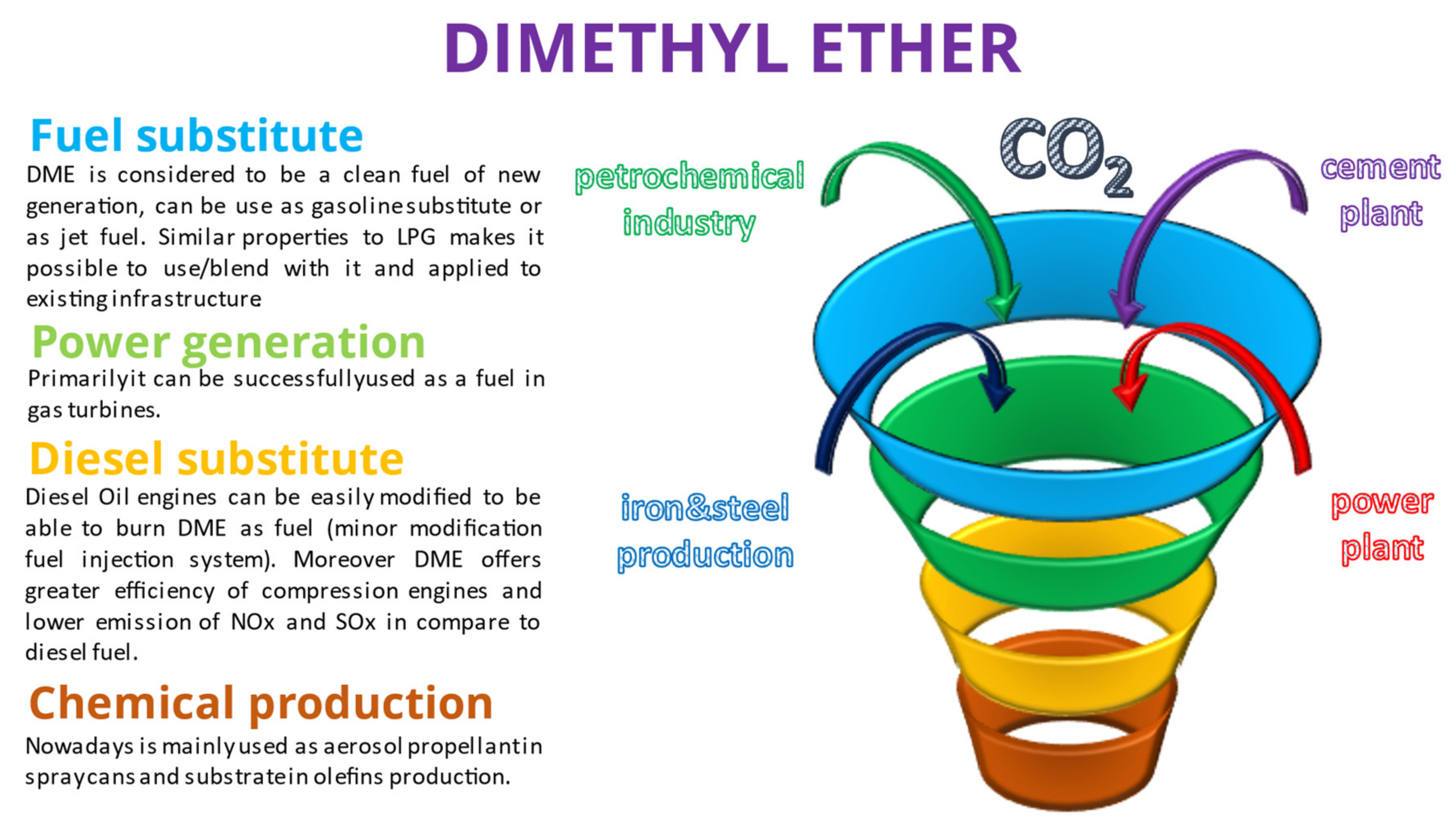
Dimethyl ether (DME) or methoxymethane is the simplest aliphatic ether with the molecular formula CH3OCH3. It is a colourless, near-odourless gas under ambient conditions. It is neither a toxic nor carcinogenic compound, with properties similar to liquid petroleum gas (LPG); thus, it can be easily blended with it and used as a fuel [9][10][11]. In the chemical industry, it is mainly used to produce diethyl sulphate, methyl acetate, light olefins and gasoline [12]. Nowadays, it is considered an alternative fuel with low emissions of NOx, hydrocarbons and carbon monoxide [2][13]. DME can be obtained in two ways: direct synthesis and through the methanol dehydration process. The first method’s reactions are:
The process of direct DME synthesis is exothermic (c.a. 246.2 kJ/mol DME); therefore, the heat produced during the reactions has to be removed. The inlet reactant mixture is composed of CO and H2.
Methanol dehydration to produce DME is also an exothermic process. CO2 can be used to produce methanol and then dehydrate it to DME. This has been proven to be a very economical way of utilizing carbon dioxide [2][14][15]. The chemical reactions occurring during the process are presented below:
Methanol formation
Methanol dehydration
Reverse water–gas shift (rWGS)
Net reaction
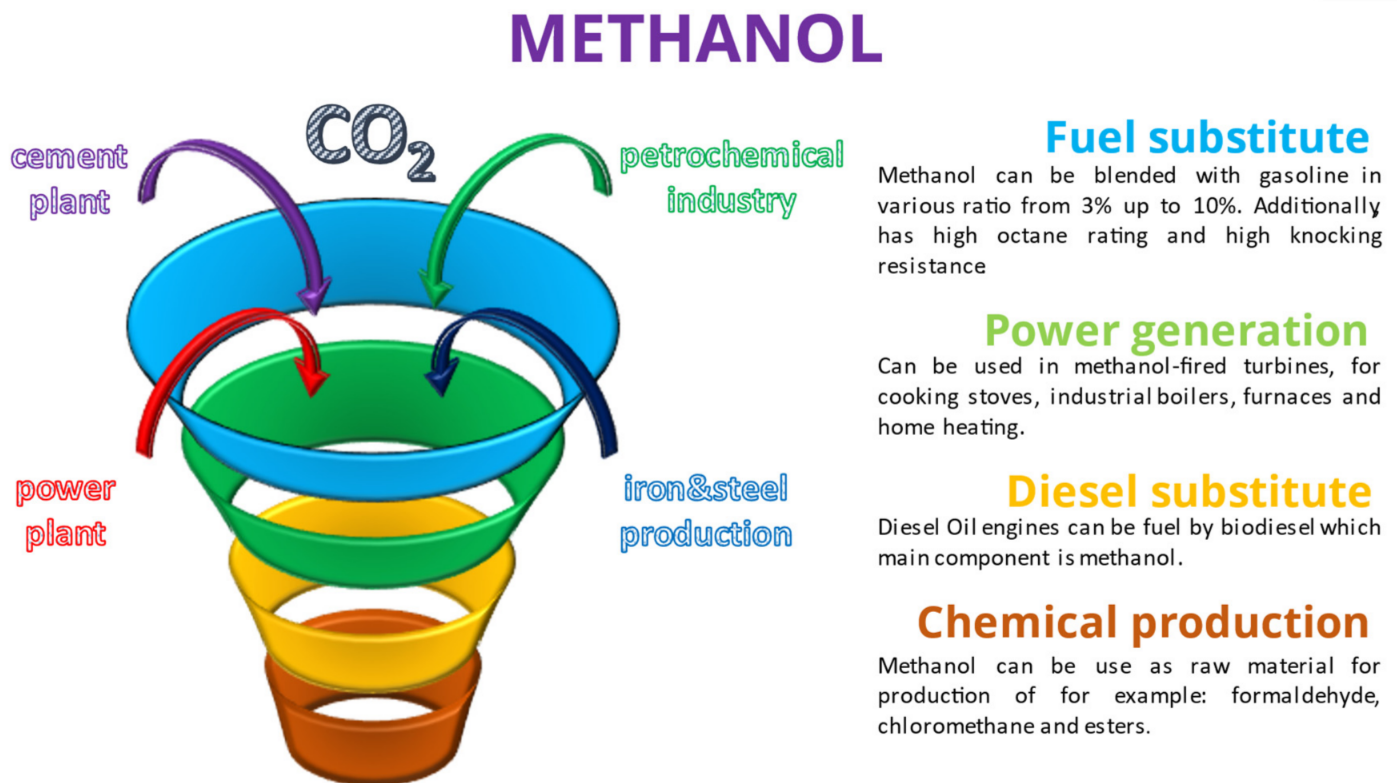
In Ref. [16], the authors explored the profitability of DME production from biogas; in Ref. [17], a techno-economic assessment of bio-DME and bio-methanol production from oil palm residue was proposed. Methanol is a colourless, flammable liquid under ambient conditions with a characteristic odour. As one of the most important raw materials, it is a substrate in many syntheses of chemical compounds, including formaldehyde, acetic acid and chloromethane. It is also a very good solvent and it is easily miscible with water, alcohols and organic solvents. Due to its wide application in many industries (fuel, chemical and other industries), the demand for methanol is constantly growing, i.e., from 47 Mt/a in 2011 [18] to 100 Mt/a in 2021 [19]. According to the report made by the International Renewable Energy Agency [19], in 2021, only 0.2% of global production of methanol came from renewable sources—more than 60% was converted by natural gas reformation and the rest was produced by natural coal gasification.
Methanol can be synthesized from a gas containing either carbon monoxide or carbon dioxide when it reacts with hydrogen:
The main properties of DME and methanol in comparison with LNG and diesel oil are shown in Table 1. Moreover, several potential major applications of DME and methanol are shown below in Figure 1 and Figure 2.
Table 1. Properties of DME and methanol compared with other fuels.
| Properties | DME [20] |
Methanol [18] |
Methane * [23] |
Diesel Fuel [24][25] |
|---|---|---|---|---|
| Molecular formula | C2H6O | CH3OH | CH4 | --- |
| CAS Number | 115-10-6 | 67-58-1 | 74-82-8 | 68334-30-5 |
| Molar mass (g/mol) | 46.07 | 32.04 | 16.04 | --- |
| Cetane number ** | 55 to 60 | ~5 | 0 | 40 to 55 |
| Melting point (°C) | −141 | −97.88 | −182.47 | −40 |
| Boiling point (°C) at 1 atm | −24.8 | 64.65 | −161.5 | 141 |
| Density at 25 °C and 1 atm (kg/m3) | 668.3 | 786.68 | 0.657 | 800–910 |
| Autoignition temperature (°C) | 235 | 440 | 537 | 250 |
| Miscible with water? | Yes | Yes | Yes | No |
| Miscible with organic solvents? *** | Yes | Yes | Yes | Yes |
* Methane as main part of Liquid Natural Gas (LNG), ** average value, *** with most popular polar and nonpolar organic solvents.
2. State-of-the-Art Production of DME/Methanol from Green Hydrogen and CO2
Converting CO2 into DME requires an energy input in the form of hydrogen, as shown in Equations (1)–(9). In order to meet the goal of reducing carbon emissions, green hydrogen is needed [1][5][26][27].
Hydrogen can be synthesized in different ways: chemical, biological, catalytic, electrochemical and thermal. To reduce the carbon footprint of the CCU process, a low-emission hydrogen production technology has to be applied. Among several possible approaches, the production of “green hydrogen” using an electrolyser powered by a renewable energy source is the most interesting [28][29].
Barbato et al. [21] presented a process for carbon dioxide conversion to green methanol where CO2 captured from a power plant was combined with hydrogen from an electrolyser powered by hydropower energy. In the calculations and conclusions, the price (with cost calculations at the prevailing rate) of methanol production resulted to be 294 €/ton and it was successfully reduced by 20% compared to the price of methanol produced traditionally.
While there are a number of pilot or demonstration-scale facilities, there are a few commercial units producing either DME or methanol on an industrial scale. Regarding existing facilities with a power plant as a carbon dioxide source, two projects developed in Germany can be taken into consideration.
The first was funded by Europe’s Horizon 2020 program (MefCO2, Project No. 637016). Nine partners established in 2014 an international cooperation to research the feasibility of CCU technology along with the production of green methanol. The main aims of the project were to demonstrate the economic feasibility of utilizing captured CO2 by converting it into a usable fuel, such as methanol, and further providing green hydrogen produced from excess energy from renewable sources. The source of carbon dioxide was a lignite-fired power plant located in Niederaussem, Germany. The project ended in 2019 with the development of one of the largest facilities in the European Union to synthesize methanol from CO2 from flue gases, capable of producing 1 ton of methanol per day while capturing more than 1.5 tons of carbon dioxide per day [30][31][32][33][34][35][36][37].
Carbon Recycling International Ltd. (CRI, Reykjavik, Iceland), known for producing renewable methanol since 2007, participated in this project. CRI’s pilot unit is located near Iceland’s capital—Reykjavik. Industrial-scale green methanol production began in 2012 in the first pilot plant with an annual capacity of about 4000 tonnes of methanol (c.a. 12 t/d). The process is based on the conversion of CO2 from geothermal sources with the hydrogen produced by water electrolysis using geothermal energy [1][36][38]. The methanol produced in this facility is used in a number of applications, including blending with gasoline, biodiesel production, and wastewater denitrification. Additionally, the CRI methanol production process reduces the environmental impact by 90% compared to conventional methods [19]. Furthermore, CRI, in cooperation with China Henan Shuncheng Group, developed in 2021 the world’s first green methanol plant with a capacity of 110,000 tons per year [39].
The ALIGN-CCUS (Project No 271501) demonstration plant is also located at Niederaussem, Germany. The project was funded through the ERA-NET ACT program and it was co-funded by the European Commission under the Horizon 2020 program ACT [40]. The source of the captured CO2 is a 1000 MW power plant unit, and the hydrogen comes from a 140 kWel alkaline electrolyser providing 22 kg of hydrogen per day. The daily production of DME is about 50 kg. For DME synthesis, a Mitsubishi Power bifunctional catalyst was used, which was responsible for both methanol synthesis and the dehydration process [41].
The post-combustion CCS is a common basis to compare new strategies to avoid carbon emissions. Olaleye et al. deeply discussed post-combustion carbon capture from a coal-fired power plant [42][43].
Blumbert et al. presented an NG-based low-pressure synthesis process for the production of methanol with CO2 utilization [44]. In their paper, the authors conducted an extended exergy analysis dividing the methanol production plant into various subsystems and evaluated the performance of each of them. Nakyai et al. [45] conducted an energy and exergy analysis of the DME production from CO and CO2 in a single-stage process. Farooqui et al. [46] evaluated from an energetic and an exergetic point of view a polygeneration plant with oxyfuel carbon capture for combined power and DME production. Researchers proposed a new configuration for DME and methanol production in a single-stage process, in which a 90% CO2 conversion rate was achieved. Researchers compared this process with post-combustion CCS and evaluated all of the chain from the NGCC power plant to the DME and methanol production. Through the exergetic analysis, researchers spotted the irreversibilities, calculated the conventional performance indicators [42][43][44] and defined new performance indicators to assess the goodness of researchers' configuration with respect to energy transformation and exergy destruction.
References
- Majchrzak-Kucęba, I.; Szymanek, P. CCU Technologies in the Green Economy. Eng. Prot. Environ. 2018, 21, 261–271.
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 31.
- Masson-Delmotte, V.P.; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.; et al. The Physical Science Basis. Contribution of Working Group I to the 552 Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021.
- Hasan, M.M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21.
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16.
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Chauvy, R.; De Weireld, G. CO2 Utilization Technologies in Europe: A Short Review. Energy Technol. 2020, 8, 2000627.
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252.
- Lee, D.; Lee, J.S.; Kim, H.Y.; Chun, C.K.; James, S.C.; Yoon, S.S. Experimental study on the combustion and no x emission characteristics of DME/LPG blended fuel using counterflow burner. Combust. Sci. Technol. 2012, 184, 97–113.
- Kim, H.J.; Suh, H.K.; Park, S.H.; Lee, C.S. An experimental and numerical investigation of atomization characteristics of biodiesel, dimethyl ether, and biodiesel-ethanol blended fuel. Energy Fuels 2008, 22, 2091–2098.
- Wang, S.; Fu, Z. Thermodynamic and economic analysis of solar assisted CCHP-ORC system with DME as fuel. Energy Convers. Manag. 2019, 186, 535–545.
- Cheng, C.; Zhang, H.; Ying, W.; Fang, D. Intrinsic kinetics of one-step dimethyl ether synthesis from hydrogen-rich synthesis gas over bi-functional catalyst. Korean J. Chem. Eng. 2011, 28, 1511–1517.
- Zhang, Y.; Zhang, S.; Benson, T. A conceptual design by integrating dimethyl ether (DME) production with tri-reforming process for CO2 emission reduction. Fuel Process. Technol. 2015, 131, 7–13.
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77.
- Bonura, G.; Todaro, S.; Frusteri, L.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Pászti, Z.; Tálas, E.; Tompos, A.; Ferenc, L.; Solt, H.; et al. Inside the reaction mechanism of direct CO2 conversion to DME over zeolite-based hybrid catalysts. Appl. Catal. B Environ. 2021, 294, 120255.
- Baena-Moreno, F.M.; Gonzalez-Castaño, M.; Arellano-García, H.; Reina, T.R. Exploring profitability of bioeconomy paths: Dimethyl ether from biogas as case study. Energy 2021, 225, 120230.
- Im-orb, K.; Arpornwichanop, A. Comparative techno-economic assessment of bio-methanol and bio-DME production from oil palm residue. Energy Convers. Manag. 2022, 258, 115511.
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Berlin, Germany, 2012.
- Kang, S.; Boshell, F.; Goeppert, A.; Prakash, S.G.; Landälv, I. Innovation Outlook: Renewable Methanol; IRENA: Abu Dhabi, United Arab Emirates, 2021.
- Müller, M.; Hübsch, U. Dimethyl Ether. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Berlin, Germany, 2000.
- Barbato, L.; Iaquaniello, G.; Mangiapane, A. Reuse of CO2 to Make Methanol Using Renewable Hydrogen. In CO2: A Valuable Source of Carbon; De Falco, M., Iaquaniello, G., Centi, G., Eds.; Green Energy and Technology; Springer: London, UK, 2013.
- Hobson, C.; Marquez, C. Renewable Methanol Report; Methanol Institute: Brussels, Belgium, 2018.
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methane (accessed on 17 February 2022).
- Trafigura. Available online: https://www.trafigura.com/media/1687/en_sds_fuel-diesel-vgo_cas-68334-30-5.pdf (accessed on 17 February 2021).
- Speight, J.G. Handbook of Petroleum Product Analysis; Wiley: Hoboken, NJ, USA, 2015; Volume 53.
- Huang, C.H.; Tan, C.S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499.
- de Falco, M.; Iaquaniello, G.; Centi, G. CO2: A Valuable Source of Carbon; Green Energy and Technology; Springer: London, UK, 2013; Volume 137.
- Scott, K. Sustainable and Green Electrochemical Science and Technology; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2016.
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732.
- RWE. Available online: https://www.rwe.com/en/press/rwe-power/2019-05-28-niederaussem-becomes-the-setting-for-important-technological-progress (accessed on 22 February 2022).
- MefCO2. Available online: http://www.mefCO2.eu/ (accessed on 21 February 2022).
- Freyr, Ó.; Magistri, L.; Licozar, B.; Diaz, A.S. Green Methanol for a Green Future. MefCO2. 2020. Available online: http://www.mefco2.eu/news/green-methanol-for-a-green-future.php (accessed on 23 February 2022).
- Fernández-González, J.; Rumayor, M.; Domínguez-Ramos, A.; Irabien, A. Hydrogen Utilization in the Sustainable Manufacture of CO2-Based Methanol. Ind. Eng. Chem. Res. 2022.
- European Commission. Available online: https://cordis.europa.eu/article/id/257671-energyintensive-sectors-can-be-part-of-low-carbon-transition (accessed on 21 February 2022).
- Ayala, P. Methanol Fuel from CO2. Synthesis of Methanol from Captured Carbon Dioxide Using Surplus Electricity. MefCO2. 13 September 2018. Available online: https://etipwind.eu/wp-content/uploads/MefCO2-slides.pdf (accessed on 23 February 2022).
- Danish Energy Agency Technology Data for Renewable Fuels. 2017. Available online: https://ens.dk/en/our-services/projections-and-models/technology-data/technology-data-renewable-fuels (accessed on 24 February 2022).
- Harp, G.; Tran, K.C.; Bergins, C.; Buddenberg, T.; Drach, I.; Sigurbjornsson, O. Application of Power to Methanol Technology to Integrated Steelworks for Profitability, Conversion Efficiency, and CO2 Reduction. In Proceedings of the METEC 2nd ESTAD, Düsseldorf, Germany, 15–19 June 2016.
- Andrei, B.D.; del Mar, P.F.M.; Evangelos, T.; Thea, S. Carbon Capture and Utilisation Workshop: Background and Proceedings; Publications Office of the European Union: Luxembourg, 2013.
- Carbon Recycling International Ltd. Available online: https://www.carbonrecycling.is/ (accessed on 22 February 2022).
- Mann, I. Deep Decarbonisation is Within Reach of Europe’s Industrial Regions. March 2021. Available online: https://www.alignccus.eu/news/align-ccus-findings-unveiled-deep-decarbonisation-within-reach-europe%E2%80%99s-industrial-regions (accessed on 24 February 2022).
- van Os, P. Accelerating low carbon industrial growth through carbon capture, utilization and storage (CCUS). Greenh. Gases Sci. Technol. 2018, 8, 994–997.
- Olaleye, A.K.; Wang, M.; Kelsall, G. Steady state simulation and exergy analysis of supercritical coal-fired power plant with CO2 capture. Fuel 2015, 151, 57–72.
- Olaleye, A.K.; Wang, M. Conventional and advanced exergy analysis of post-combustion CO2 capture based on chemical absorption integrated with supercritical coal-fired power plant. Int. J. Greenh. Gas Control 2017, 64, 246–256.
- Blumberg, T.; Morosuk, T.; Tsatsaronis, G. Exergy-based evaluation of methanol production from natural gas with CO2 utilization. Energy 2017, 141, 2528–2539.
- Nakyai, T.; Saebea, D. Energy and exergy analyses of dimethyl ether production using CO and CO2 hydrogenation. Int. J. Smart Grid Clean Energy 2019, 8, 544–549.
- Farooqui, A.; Di Tomaso, F.; Bose, A.; Ferrero, D.; Llorca, J.; Santarelli, M. Techno-economic and exergy analysis of polygeneration plant for power and DME production with the integration of chemical looping CO2/H2O splitting. Energy Convers. Manag. 2019, 186, 200–219.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
2 times
(View History)
Update Date:
31 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No