You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | zanlin chen | -- | 2904 | 2022-05-26 06:39:01 | | | |
| 2 | Dean Liu | -19 word(s) | 2885 | 2022-05-31 02:43:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, Z.; Xie, M.; , . Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor. Encyclopedia. Available online: https://encyclopedia.pub/entry/23394 (accessed on 21 December 2025).
Chen Z, Xie M, . Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor. Encyclopedia. Available at: https://encyclopedia.pub/entry/23394. Accessed December 21, 2025.
Chen, Zanlin, Miaojia Xie, . "Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor" Encyclopedia, https://encyclopedia.pub/entry/23394 (accessed December 21, 2025).
Chen, Z., Xie, M., & , . (2022, May 26). Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor. In Encyclopedia. https://encyclopedia.pub/entry/23394
Chen, Zanlin, et al. "Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor." Encyclopedia. Web. 26 May, 2022.
Copy Citation
The electrochemical aptamer sensor (E-apt sensor), which is composed of biometric elements and signal sensors, has attracted more and more attention for this purpose. The signal sensor usually consists of an electrode substrate, modified layer, and electrochemical signal detection system. The most widely used electrode substrates include a gold electrode (AuE), glassy carbon electrode (GCE), indium tin oxide electrode (ITO), reduced graphene electrode (ERGO), and screen-printed electrode (SPE). Nanomaterials are particularly important in the construction of E-apt sensors, which can be used as aptamer carriers or sensitizers to stimulate or inhibit electrochemical signals.
heavy metal ions
aptamer
electrochemical biosensor
nanomaterials
1. Introduction
With the rapid development of industry and the improvement of urbanization, more and more chemical substances are used in daily life and agricultural production. Increasingly frequent industrial activities such as mining, metallurgy, and oil extraction produces many toxic and harmful substances. These toxic and harmful substances, even after purification treatment, will still leave some residues in the natural water system, including heavy metals, inorganic salts, and agricultural veterinary drugs, which cause pollution and damage the water environment [1][2][3]. Unlike organic pollutants, heavy metals cannot be biodegraded under natural conditions [4] and will be passively ingested by plants through drinking and irrigation, and eventually, will enter the human body through continuous accumulation in the food chain. Mercury, cadmium, lead, Chromium, Thallium, Antimony, and arsenic are the most common heavy metal pollutants. According to WHO standards, they usually do not exceed 2 ppb. The heavy metals ingested into the human body are likely to form complexes with biological substances such as proteins, enzymes, and nucleic acids. The formation of such complexes alters the molecular composition and mechanism of biological matter, causing it to fail to perform its original physiological function or causing distortion [5]. The accumulation of these elements can cause serious damage to the gut, bones, central nervous system, liver, kidneys, and reproductive system. Since these elements cannot be removed by normal removal methods, even trace amounts of heavy metals can pose a serious threat to living things [6].
In these cases, detection of heavy metal ions in environmental and water systems to prevent heavy metal pollution from the source of the food chain is a vital need. In recent years, many detection methods for heavy metal ions have been developed. Traditional detection methods mainly calculate the concentration of an atom based on its characteristic spectral intensity, including atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectroscopy (ICP-MS), X-ray fluorescence spectrometry (XRF), neutron activation analysis (NAA), and inductively coupled plasma-atomic emission spectrometry (ICP-AES) [7]. These methods can perform accurate qualitative and quantitative analyses of heavy metal ions with high sensitivity, but they are also expensive and require laborious pre-processing [8]. Therefore, a cost-effective, fast, and efficient detection method for heavy metal ions needs to be developed.
Biosensors play an indispensable role in the development of biotechnology and are a fast analytical tool for detection at the molecular level. Usually, the recognition factor used in this technology is an antibody, but the emergence of the aptamer brings new prospects and possibilities to the field of biosensor analysis [9]. An aptamer is an artificial, single-stranded oligomer probe of DNA and RNA consisting of 10–100 bases, obtained by the SELEX index enrichment method. In Figure 1 the general process of SELEX is shown in detail. The initial ssDNA library was exposed in a container filled with target molecules, and the sequences with specific binding ability to the target were separated from the library. Then, a PCR reaction was used for bulk amplification and the next round of screening was carried out until ssDNA with the highest affinity to the target was obtained. Aptamers can fold into complex structures, selectively binding to the target with high affinity and specificity, known as artificial antibodies [10][11][12][13]. Compared with antibodies, aptamers have obvious advantages such as high chemical stability, low cost, easy operation, and they are easy to obtain [14]. Aptamers have a wide range of recognition targets, such as proteins, small molecules, agricultural veterinary drugs, bacteria, and heavy metal ions [15][16][17][18][19], which have broad application prospects in the field of food detection. A variety of biosensors using aptamers as recognition factors have been developed and applied to the detection of heavy metal ions, providing a new, efficient, and fast platform for the detection of heavy metal ions.
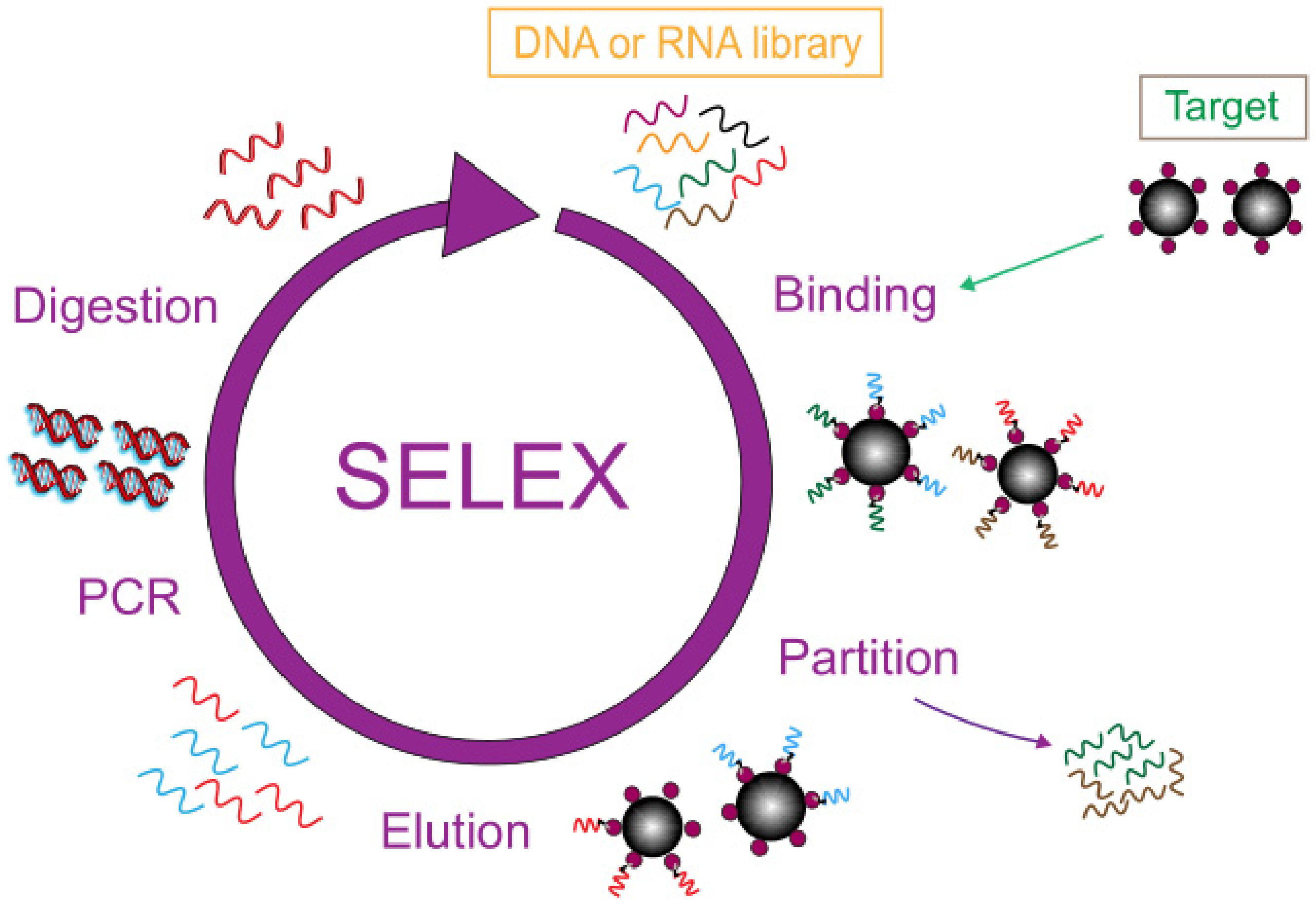
Figure 1. SELEX flowchart.
The electrochemical aptamer sensor (E-apt sensor), which is composed of biometric elements and signal sensors, has attracted more and more attention for this purpose. The signal sensor usually consists of an electrode substrate, modified layer, and electrochemical signal detection system. The most widely used electrode substrates include a gold electrode (AuE), glassy carbon electrode (GCE), indium tin oxide electrode (ITO), reduced graphene electrode (ERGO), and screen-printed electrode (SPE). Different electrode materials can have different degrees of signal enhancement after being modified by an appropriately modified layer and a variety of nanomaterials can be selected for the modified layer. The aptamer is fixed on the surface of the electrode by intermolecular force with the modified layer. This operation will change the impedance of the electrode and cause a current change. Therefore, nanomaterial modification in electrodes becomes an important part of electrochemical sensor construction. The electrochemical signal detection system includes a signal amplifier, a processor, and a display screen. The electrochemical sensor has been widely used in recent years because of its advantages of simplicity, high efficiency, strong specificity, and high sensitivity. As shown in Figure 2, the number of papers and patents published with the keywords “Electrochemical” and “Aptamer” has increased year by year since 2012 (data for 2022 is up to March).
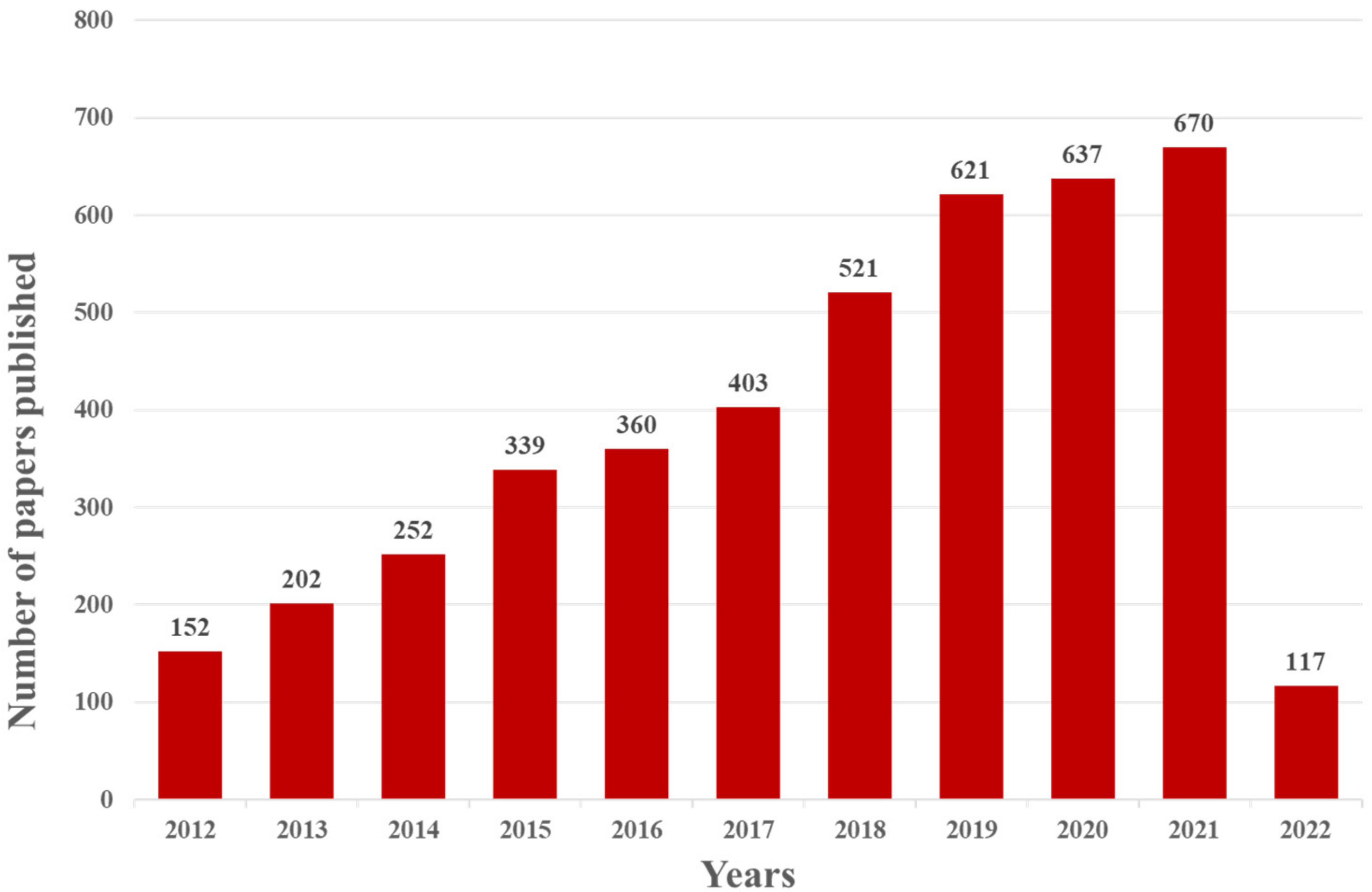
Figure 2. Web of Science report for the number of indexed papers and patents about the application of the E-apt sensor (keyword: “Electrochemical” and “Aptamer”.
2. Application of Nanomaterials in Aptamer-Based Electrochemical Sensors
In recent years, the successful synthesis of various nanomaterials has attracted significant attention. Their unique physical and chemical properties, including high surface area/volume ratio, high reactivity, size dependence, and high functionalization, make them widely used in chemical analysis, therapy, diagnosis, and food safety [20][21][22][23][24]. In addition to the binding activity of the aptamer, the reason why the electrochemical aptamer sensors can detect heavy metal ions as low as fM is largely attributed to the rational use of nanomaterials. Sensing platforms are formed by immobilizing aptamers on nanomaterial surfaces through intermolecular forces or catalyzing chemical reactions in sensors as sensitizers to enhance electrical signals and improve the specificity and sensitivity of sensors [25]. Due to their recognized properties, the coupling of aptamers on nanomaterials has great potential to form biosensor platforms.
Gold nanoparticles, carbon nanotubes, graphene, quantum dots, and metal-organic frameworks are common and basic nanomaterials used in E-apt sensors. These materials can be mixed or coupled with other substances to create new composite materials. They have shown many advantages such as high specific surface area, good biocompatibility, high electrical conductivity, high magnetic properties, and unique electro-optic and physicochemical properties [26][27][28]. In addition to being used as sensitizers, most nanomaterials in E-apt sensors are used to fix aptamers to form couplings with a better capture effect. Researchers often use couplings as probes to obtain better detection results. Figure 3 indicates how popular nanomaterials (shown as their microscopic morphology) are coupled with aptamers.
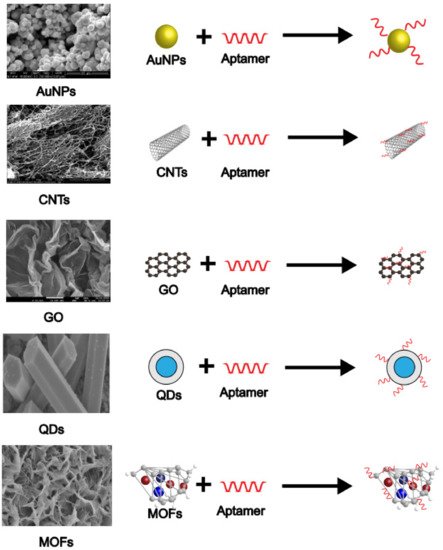
Figure 3. SEM images and combination modes of various nanomaterials.
2.1. Gold Nanoparticles
Among the aptamer-based sensors developed, metal nanoparticles such as gold nanoparticles (AuNPs) are the most common advanced materials. They have high stability and oxidation resistance and can be prepared through various physical and chemical methods, among which the most classical preparation method was proposed by Turkevich et al. in 1951 [29]. The high surface-volume ratio and excellent optical properties of AuNPs contribute to the high sensitivity and selectivity of the target detection. The optical properties of AuNPs are largely dependent on the size, shape, and aggregation state of nanoparticles. Colloidal AuNPs are usually red or pink and turn purplish-blue when aggregated, forming the basis of colorimetric detection [30]. AuNPs are also conductive and fluorescent and, thus, have the ability to respond to external electrochemical and optical stimulation.
One of the most common approaches to using AuNPs in electrochemical sensors is to anchor aptamers. The aptamer is usually fixed by the force of the Au-S bond; Yuan et al. [31] used this method to develop a sensor for detecting Pb2+ and Cd2+ at the same time. Due to the specific binding of aptamers to metal ions, methylene blue or ferrocene labeled aptamers were competitively separated from the gold electrode, resulting in a weaker electrochemical signal. The detection limits of Cd2+ and Pb2+ were 89.31 and 16.44 pM. Deng et al. [32] deposited L-cysteine and AuNPs layers on the electrode surface successively providing a large surface area to anchor many sulfur-capped auxiliary probes through the mercaptan-gold interaction. Additionally, to fix the aptamer, Liu et al. used chitosan to attach AuNPs to the electrode to enhance electron transfer, thus improving the sensitivity of the sensor [33].
In addition to being used alone, AuNPs are also used as composite materials coupled with other metal ions. The composite materials tend to enhance the electrochemical signal so that even subtle changes can be detected. Silver and gold alloy nanoparticles (Ag-Au alloy NPs) promise to create inexpensive and stable electrochemical sensors [34]. This is because silver-gold alloy nanoparticles have a large specific surface area and good electrical conductivity which can act as a conductive center and promote electron transfer, thus trapping more heavy metal ions on the electrode. Zhao et al. [35] used DNA enzyme functionalized (Ag-Au alloy) NPs to form core-shell nanoparticles, which can be used as signal tags. Because the core-shell structure increases the specific surface area and catalyzes the reaction, the electrochemical signal can be enhanced. Ag-Au Alloy NPs were also used to modify GCE and the resulting sensor had higher sensitivity and reproducibility. Miao et al. [36] used Fe3O4@AuNPs to carry a DNA probe, and the other two were labeled with independent electrochemical substances which can detect both Hg2+ and Ag2+. Due to their excellent signal amplification, AuNPs have been one of the most used transduction materials to construct E-apt sensors for the analysis of food and water contaminants.
2.2. Carbon Nanotubes
Carbon nanotubes (CNTs) are a kind of carbon nanomaterial with unique electrical transmission properties and a large specific surface area, with excellent chemical, mechanical, and thermal stability, and direct binding with other molecules. Therefore, they have received great attention in biosensor design [37][38]. They can be thought of as cylindrical tubes made of one or more sheets of graphene rolled and folded. Nanotubes formed from a single sheet of graphene are called single-walled nanotubes (SWNT), with diameters between 0.4~2 nm, and multi-walled carbon nanotubes(MWCNTs) are 2~10 times larger [39]. Graphene contains SP2 hybrid carbon atoms, and nucleic acids can easily be attached to the surface of the nanotubes through π-π bonds, so CNTs are often used to carry ssDNA or RNA. In 2005, So et al. [40] constructed a biosensor using single-walled CNTs by using aptamer as a molecular recognition element for the first time. Since then, the potential of CNTs in sensing platforms has been gradually explored.
It is more common to modify electrodes with MWCNTs and then fix aptamers on the surface of MWCNTs. Zhu et al. [41] used carboxylic acid group functionalized MWCNTs to fix aptamers. Because the material has a large surface area and good charge transferability, the DNA attachment amount and sensor performance could be significantly improved. The sensor could detect Pb2+ in the range of 5.0 × 10−11~1.0 × 10−14 M. Additionally, Zou et al. [42] covalently immobilized aptamers on carboxylic functionalized MWCNTs by EDC/NHS chemistry. CNTs can not only carry aptamers in sensors but also provide binding sites for the reactions between aptamers and other nanomaterials. Rabai et al. [43] modified the electrode surface with CNTs, then deposited AuNPs on the composite electrode. Finally, with the aptamer fixed, the sensor had a detection limit of 0.02 pM for Cd2+. He et al. [44] developed an electrochemical sensor with a Zn3(PO4)2 modified aptamer and found that the sensor with MWCNTs had higher sensitivity.
2.3. Graphene
Graphene is a two-dimensional carbon nanomaterial with SP2 hybrid connected carbon atoms tightly packed into a single layer honeycomb lattice structure. Due to its excellent electrical properties, graphene has attracted great interest in recent years. Graphene has a unique type of structure that also gives it features not found in many other nanomaterials; its large specific surface area makes it an ideal candidate for immobilizing large numbers of functionalized metal oxides and noble metal nanoparticles [45]. Exceptional carrier mobility offers great prospects for nanoscale applications, such as electronic devices and chemical/biological sensors. In addition, its high electron transfer efficiency and wide electrochemical window make graphene an ideal material for constructing a highly sensitive E-apt sensor [46]. In recent years, graphene as a REDOX molecule in electrochemical sensors has attracted great attention.
The most common use of graphene in sensors is to modify electrodes. Zhang et al. [47] developed an Hg2+ detector for a graphene-fixed aptamer probe with a detection limit of 5 pM. Using graphene-modified electrodes, the modified electrode surface can be reused. Similar to AuNPs, it is more likely that graphene will be coupled with other nanomaterials to form new composite materials for better modification. Hai et al. [48] modified the electrode with AuNPs coupled with graphene. The aptamer was self-assembled on the electrode, and the detection limit of Pb2+ was as low as 3.8 pM. Jiang et al. [49] designed a new nanometer composite material consisting of TiO2, AuNPs, and nitrogen-doped graphene. The composite exhibits excellent optical properties, increasing the exciton lifetime and improving the charge transfer photocurrent intensity to 18.2 times higher than that of original TiO2. Wang et al. [50] synthesized another new material modified electrode by using CS instead of AuNPs for the detection of Pb2+. The modified electrode showed good repeatability, stability, and specificity to other interfering metal ions. Li et al. [51] prepared a sensor for mercury ions based on a perylene-3, 4, 9, 10-tetracarboxylic acid/graphene oxide (PTCA/GO) and quercetin-copper(II) complex. They efficiently promoted the separation of photoexcited carriers and enhanced the photocurrent.
2.4. Quantum Dots
Quantum dots (QDs), also known as semiconductor nanocrystals, are mixtures of cadmium and selenium or tellurium with nanoscale clusters with diameters of 1–20 nm [52]. Due to their unique properties, such as high quantum yield, excitation-dependent emission, surface modification versatility, and long-term photostability [53][54], QDs have been used in many research fields, especially in nanoelectronics, optoelectronics, and biological analysis [55]. The physical size of the nanocrystals determines the wavelength of the emitted fluorescence, so multiple analyses can be performed using a single excitation source. With the rapid development of nanotechnology, such nanomaterials have been widely used to improve the sensitivity of sensors. When they are incorporated into the design of biosensors, they can be used as tags, as part of a signal sensor [56].
QDs are mainly used in electrochemical luminescence sensors and photoelectric electrochemistry sensors. When the aptamer reacts with the corresponding target, the brightness of the QDs will also change due to the resonance energy transfer, thus improving the sensitivity of the sensor. CdTe QDs are most frequently used in sensors; Shi et al. [57] developed a new method for the detection of Pb2+ based on the sensitization effect of CdTe QDs. When the target is present, the labeled QDs close to the electrode surface produce a sensitization effect and the photocurrent intensity is enhanced. Feng et al. [58] modified CdTe QDs with MIL-53 and determined Hg2+ and Pb2+ simultaneously by the ECL method with good recovery. Except for CdTe QDs, there are many other types of QDs, such as CdS and nitrogen-doped graphene (NG) [59][60], and their principle of action is roughly the same. When the conformation of the aptamer changes, the distance between the QDs and the electrode alters, and the electrical signal is either enhanced or weakened. This property makes QDs widely used to design E-apt sensors for food and water analysis.
2.5. Metal-Organic Frameworks
More and more metal-organic frameworks (MOFs) have been found to effectively coordinate polymer nanomaterials and have received extensive attention as effective quenching materials. MOFs are a kind of crystalline nanomaterial composed of metal ions and organic ligands. Due to their water dispersibility, adjustability, biocompatibility, low cost, controllable shape, ultra-high porosity, and high specific surface area [61], MOFs have been increasingly used in biosensors, electrocatalysis, energy storage, and conversion. The large specific surface area and ultra-high porosity of MOFs provide more reaction sites for aptamers and targets. Organic ligands with rich functional groups make MOFs easy to be functionalized with various molecules and materials [62]. In addition, MOF compositions take a variety of forms (e.g., nanosheets, cages, tubes, rods, cubes, etc.) and can be easily adjusted according to the selection of various organic connectives and metal ions [63].
Aptamers are securely fixed in the MOFs by encapsulation. The main framework of MOFs can facilitate various interactions with analytes through functional groups in organic ligands, thus achieving high sensitivity and high selectivity recognition. Therefore, MOFs can be used as signal probes for different detection methods. Zhang et al. [64] used a Zr-based MOF embedded with three kinds of aptamer. Ling et al. [65] prepared streptavidin functionalized zirconium porphyrin MOF (PCN-222@SA) using a covalent method as a signal nanoprobe. Introducing this signal nanoprobe into the sensor surface significantly amplified the electrocatalytic current. Zhang et al. [66] synthesized a core-shell nanostructured composite material composed of Fe (III)-based MOF (Fe-MOF) and mesoporous Fe3O4@C nanocapsules (Fe-MOF@mFe3O4 @MC) that exhibited excellent electrochemical activity, water stability, and high specific surface area, resulting in strong biological binding to heavy metal ion targeting aptamer chains.
References
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Efremova Aaron, S.; Aaron, J.-J. Toxic Heavy Metals: Impact on the Environment and Human Health, and Treatment with Conducting Organic Polymers, a Review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942.
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy Metal Contamination Status in Greek Surface Waters: A Review with Application and Evaluation of Pollution Indices. Chemosphere 2021, 263, 128192.
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595.
- Fakhri, Y.; Saha, N.; Miri, A.; Baghaei, M.; Roomiani, L.; Ghaderpoori, M.; Taghavi, M.; Keramati, H.; Bahmani, Z.; Moradi, B.; et al. Metal Concentrations in Fillet and Gill of Parrotfish (Scarus Ghobban) from the Persian Gulf and Implications for Human Health. Food Chem. Toxicol. 2018, 118, 348–354.
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458.
- Al Hamouz, O.C.S.; Akintola, O.S. Removal of Lead and Arsenic Ions by a New Series of Aniline Based Polyamines. Process Saf. Environ. Prot. 2017, 106, 180–190.
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521.
- Harrington, C.F.; Clough, R.; Drennan-Harris, L.R.; Hill, S.J.; Tyson, J.F. Atomic Spectrometry Update. Elemental Speciation. J. Anal. At. Spectrom. 2011, 26, 1561.
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent Advances in Aptamer-Based Optical and Electrochemical Biosensors for Detection of Pesticides and Veterinary Drugs. Food Control 2022, 131, 108399.
- Li, F.; Yu, Z.; Han, X.; Lai, R.Y. Electrochemical Aptamer-Based Sensors for Food and Water Analysis: A Review. Anal. Chim. Acta 2019, 1051, 1–23.
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three Decades of Nucleic Acid Aptamer Technologies: Lessons Learned, Progress and Opportunities on Aptamer Development. Biotechnol. Adv. 2019, 37, 28–50.
- Zhu, G.; Chen, X. Aptamer-Based Targeted Therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78.
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519.
- Wang, T.; Chen, L.; Chikkanna, A.; Chen, S.; Brusius, I.; Sbuh, N.; Veedu, R.N. Development of Nucleic Acid Aptamer-Based Lateral Flow Assays: A Robust Platform for Cost-Effective Point-of-Care Diagnosis. Theranostics 2021, 11, 5174–5196.
- Wu, S.; Zhang, H.; Shi, Z.; Duan, N.; Fang, C.; Dai, S.; Wang, Z. Aptamer-Based Fluorescence Biosensor for Chloramphenicol Determination Using Upconversion Nanoparticles. Food Control 2015, 50, 597–604.
- Emrani, A.S.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric and Fluorescence Quenching Aptasensors for Detection of Streptomycin in Blood Serum and Milk Based on Double-Stranded DNA and Gold Nanoparticles. Food Chem. 2016, 190, 115–121.
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A Highly Sensitive Detection of Carbendazim Pesticide in Food Based on the Upconversion-MnO2 Luminescent Resonance Energy Transfer Biosensor. Food Chem. 2021, 349, 129157.
- You, H.; Bai, L.; Yuan, Y.; Zhou, J.; Bai, Y.; Mu, Z. An Amperometric Aptasensor for Ultrasensitive Detection of Sulfadimethoxine Based on Exonuclease-Assisted Target Recycling and New Signal Tracer for Amplification. Biosens. Bioelectron. 2018, 117, 706–712.
- Zhou, C.; Zou, H.; Sun, C.; Ren, D.; Xiong, W.; Li, Y. Fluorescent Aptasensor for Detection of Four Tetracycline Veterinary Drugs in Milk Based on Catalytic Hairpin Assembly Reaction and Displacement of G-Quadruplex. Anal. Bioanal. Chem. 2018, 410, 2981–2989.
- Chen, Q.; Sheng, R.; Wang, P.; Ouyang, Q.; Wang, A.; Ali, S.; Zareef, M.; Hassan, M.M. Ultra-Sensitive Detection of Malathion Residues Using FRET-Based Upconversion Fluorescence Sensor in Food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118654.
- Dolati, S.; Ramezani, M.; Nabavinia, M.S.; Soheili, V.; Abnous, K.; Taghdisi, S.M. Selection of Specific Aptamer against Enrofloxacin and Fabrication of Graphene Oxide Based Label-Free Fluorescent Assay. Anal. Biochem. 2018, 549, 124–129.
- Zhang, S.; Geryak, R.; Geldmeier, J.; Kim, S.; Tsukruk, V.V. Synthesis, Assembly, and Applications of Hybrid Nanostructures for Biosensing. Chem. Rev. 2017, 117, 12942–13038.
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2018, 7, 1700889.
- Baig, N.; Sajid, M.; Saleh, T.A. Recent Trends in Nanomaterial-Modified Electrodes for Electroanalytical Applications. TrAC Trends Anal. Chem. 2019, 111, 47–61.
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor Based on Fluorophore-Quencher Nano-Pair and Smartphone Spectrum Reader for on-Site Quantification of Multi-Pesticides. Biosens. Bioelectron. 2018, 117, 75–83.
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Hashemi Fath, R. Impedimetric Ultrasensitive Detection of Chloramphenicol Based on Aptamer MIP Using a Glassy Carbon Electrode Modified by 3-Ampy-RGO and Silver Nanoparticle. Colloids Surf. B Biointerfaces 2019, 183, 110451.
- Charbgoo, F.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Nanoparticles Application in High Sensitive Aptasensor Design. TrAC Trends Anal. Chem. 2016, 85, 85–97.
- Fernandes, P.M.V.; Campiña, J.M.; Silva, A.F. A Layered Nanocomposite of Laccase, Chitosan, and Fe3O4 Nanoparticles-Reduced Graphene Oxide for the Nanomolar Electrochemical Detection of Bisphenol A. Microchim. Acta 2020, 187, 262.
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55.
- Priyadarshini, E.; Pradhan, N. Gold Nanoparticles as Efficient Sensors in Colorimetric Detection of Toxic Metal Ions: A Review. Sens. Actuators B Chem. 2017, 238, 888–902.
- Yuan, M.; Qian, S.; Cao, H.; Yu, J.; Ye, T.; Wu, X.; Chen, L.; Xu, F. An Ultra-Sensitive Electrochemical Aptasensor for Simultaneous Quantitative Detection of Pb2+ and Cd2+ in Fruit and Vegetable. Food Chem. 2022, 382, 132173.
- Deng, W.; Hong, L.-R.; Zhao, M.; Zhuo, Y.; Gao, M. Electrochemiluminescence-Based Detection Method of Lead(ii) Ion via Dual Enhancement of Intermolecular and Intramolecular Co-Reaction. Analyst 2015, 140, 4206–4211.
- Liu, Y.; Lai, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S.; Wang, Z. Cd-Aptamer Electrochemical Biosensor Based on AuNPs/CS Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259.
- Yadav, R.; Kushwah, V.; Gaur, M.S.; Bhadauria, S.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Electrochemical Aptamer Biosensor for As 3+ Based on Apta Deep Trapped Ag-Au Alloy Nanoparticles-Impregnated Glassy Carbon Electrode. Int. J. Environ. Anal. Chem. 2020, 100, 623–634.
- Zhao, Y.; Xie, X. A Novel Electrochemical Aptamer Biosensor Based on DNAzyme Decorated Core-Shell Nanoparticles for Hg2+ Determination. J. Braz. Chem. Soc. 2018, 29, 232–239.
- Miao, P.; Tang, Y.; Wang, L. DNA Modified Fe3O4 @Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947.
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800.
- Charbgoo, F.; Behmanesh, M.; Nikkhah, M. Enhanced Reduction of Single-Wall Carbon Nanotube Cytotoxicity in Vitro: Applying a Novel Method of Arginine Functionalization. Biotechnol. Appl. Biochem. 2015, 62, 598–605.
- Wilder, J.W.G.; Venema, L.C.; Rinzler, A.G.; Smalley, R.E.; Dekker, C. Electronic Structure of Atomically Resolved Carbon Nanotubes. Nature 1998, 391, 59–62.
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc. 2005, 127, 11906–11907.
- Zhu, Y.; Zeng, G.; Zhang, Y.; Tang, L.; Chen, J.; Cheng, M.; Zhang, L.; He, L.; Guo, Y.; He, X.; et al. Highly Sensitive Electrochemical Sensor Using a MWCNTs/GNPs-Modified Electrode for Lead (II) Detection Based on Pb 2+ -Induced G-Rich DNA Conformation. Analyst 2014, 139, 5014.
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194.
- Rabai, S.; Teniou, A.; Catanante, G.; Benounis, M.; Marty, J.-L.; Rhouati, A. Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water. Sensors 2021, 22, 105.
- He, L.; Zhang, S.; Wang, M.; Peng, D.; Yan, F.; Zhang, Z.; Zhou, L. Facile Fabrication of Zinc Phosphate-Based Nanocomposites for High-Performance Electrochemical Sensing of Hg(II). Sens. Actuators B Chem. 2016, 228, 500–508.
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600.
- Biswas, C.; Lee, Y.H. Graphene Versus Carbon Nanotubes in Electronic Devices. Adv. Funct. Mater. 2011, 21, 3806–3826.
- Zhang, Y.; Xie, J.; Liu, Y.; Pang, P.; Feng, L.; Wang, H.; Wu, Z.; Yang, W. Simple and Signal-off Electrochemical Biosensor for Mercury(II) Based on Thymine-Mercury-Thymine Hybridization Directly on Graphene. Electrochim. Acta 2015, 170, 210–217.
- Hai, H.; Yang, F.; Li, J. Highly Sensitive Electrochemiluminescence “Turn-on” Aptamer Sensor for Lead(II) Ion Based on the Formation of a G-Quadruplex on a Graphene and Gold Nanoparticles Modified Electrode. Microchim. Acta 2014, 181, 893–901.
- Jiang, D.; Du, X.; Chen, D.; Zhou, L.; Chen, W.; Li, Y.; Hao, N.; Qian, J.; Liu, Q.; Wang, K. One-Pot Hydrothermal Route to Fabricate Nitrogen Doped Graphene/Ag-TiO2: Efficient Charge Separation, and High-Performance “on-off-on” Switch System Based Photoelectrochemical Biosensing. Biosens. Bioelectron. 2016, 83, 149–155.
- Wang, L.; Peng, X.; Fu, H. An Electrochemical Aptasensor for the Sensitive Detection of Pb2+ Based on a Chitosan/Reduced Graphene Oxide/Titanium Dioxide. Microchem. J. 2022, 174, 106977.
- Li, H.; Xue, Y.; Wang, W. Femtomole Level Photoelectrochemical Aptasensing for Mercury Ions Using Quercetin–Copper(II) Complex as the DNA Intercalator. Biosens. Bioelectron. 2014, 54, 317–322.
- Esteve-Turrillas, F.A.; Abad-Fuentes, A. Applications of Quantum Dots as Probes in Immunosensing of Small-Sized Analytes. Biosens. Bioelectron. 2013, 41, 12–29.
- Shahdost-fard, F.; Roushani, M. Designing an Ultra-Sensitive Aptasensor Based on an AgNPs/Thiol-GQD Nanocomposite for TNT Detection at Femtomolar Levels Using the Electrochemical Oxidation of Rutin as a Redox Probe. Biosens. Bioelectron. 2017, 87, 724–731.
- Khonsari, Y.N.; Sun, S. A Novel Label Free Electrochemiluminescent Aptasensor for the Detection of Lysozyme. Mater. Sci. Eng. C 2019, 96, 146–152.
- Cao, J.-T.; Liao, X.-J.; Wang, Y.-L.; Liu, Y.-M. A Novel Photoelectrochemical Strategy for Lead Ion Detection Based on CdSe Quantum Dots Co-Sensitized ZnO-CdS Nanostructure. J. Electroanal. Chem. 2021, 880, 114828.
- Adegoke, O.; Daeid, N.N. Alloyed AuFeZnSe Quantum Nanorod Nanocomposite as an Ultrasensitive and Selective Plasmon-Amplified Fluorescence OFF-ON Aptasensor for Arsenic (III). J. Photochem. Photobiol. A Chem. 2022, 426, 113755.
- Shi, J.-J.; Zhu, J.-C.; Zhao, M.; Wang, Y.; Yang, P.; He, J. Ultrasensitive Photoelectrochemical Aptasensor for Lead Ion Detection Based on Sensitization Effect of CdTe QDs on MoS2-CdS:Mn Nanocomposites by the Formation of G-Quadruplex Structure. Talanta 2018, 183, 237–244.
- Feng, D.; Li, P.; Tan, X.; Wu, Y.; Wei, F.; Du, F.; Ai, C.; Luo, Y.; Chen, Q.; Han, H. Electrochemiluminescence Aptasensor for Multiple Determination of Hg2+ and Pb2+ Ions by Using the MIL-53(Al)@CdTe-PEI Modified Electrode. Anal. Chim. Acta 2020, 1100, 232–239.
- Li, L.; Chen, B.; Luo, L.; Liu, X.; Bi, X.; You, T. Sensitive and Selective Detection of Hg2+ in Tap and Canal Water via Self-Enhanced ECL Aptasensor Based on NH2– Talanta 2021, 222, 121579.
- Zang, Y.; Lei, J.; Hao, Q.; Ju, H. “Signal-On” Photoelectrochemical Sensing Strategy Based on Target-Dependent Aptamer Conformational Conversion for Selective Detection of Lead(II) Ion. ACS Appl. Mater. Interfaces 2014, 6, 15991–15997.
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2021, 31, 2006970.
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-Functionalized Metal-Organic Frameworks (MOFs) for Biosensing. Biosens. Bioelectron. 2021, 176, 112947.
- Pavadai, R.; Amalraj, A.; Subramanian, S.; Perumal, P. High Catalytic Activity of Fluorophore-Labeled Y-Shaped DNAzyme/3D MOF-MoS 2 NBs as a Versatile Biosensing Platform for the Simultaneous Detection of Hg 2+, Ni 2+, and Ag + Ions. ACS Appl. Mater. Interfaces 2021, 13, 31710–31724.
- Zhang, Z.-H.; Duan, F.-H.; Tian, J.-Y.; He, J.-Y.; Yang, L.-Y.; Zhao, H.; Zhang, S.; Liu, C.-S.; He, L.-H.; Chen, M.; et al. Aptamer-Embedded Zirconium-Based Metal–Organic Framework Composites Prepared by De Novo Bio-Inspired Approach with Enhanced Biosensing for Detecting Trace Analytes. ACS Sens. 2017, 2, 982–989.
- Ling, P.; Lei, J.; Ju, H. Porphyrinic Metal-Organic Framework as Electrochemical Probe for DNA Sensing via Triple-Helix Molecular Switch. Biosens. Bioelectron. 2015, 71, 373–379.
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.-Y.; He, L.; Zhang, X.; Liu, C.-S. Fe(III)-Based Metal–Organic Framework-Derived Core–Shell Nanostructure: Sensitive Electrochemical Platform for High Trace Determination of Heavy Metal Ions. Biosens. Bioelectron. 2017, 94, 358–364.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
31 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




