The electrochemical aptamer sensor (E-apt sensor), which is composed of biometric elements and signal sensors, has attracted more and more attention for this purpose. The signal sensor usually consists of an electrode substrate, modified layer, and electrochemical signal detection system. The most widely used electrode substrates include a gold electrode (AuE), glassy carbon electrode (GCE), indium tin oxide electrode (ITO), reduced graphene electrode (ERGO), and screen-printed electrode (SPE). Nanomaterials are particularly important in the construction of E-apt sensors, which can be used as aptamer carriers or sensitizers to stimulate or inhibit electrochemical signals.
- heavy metal ions
- aptamer
- electrochemical biosensor
- nanomaterials
1. Introduction
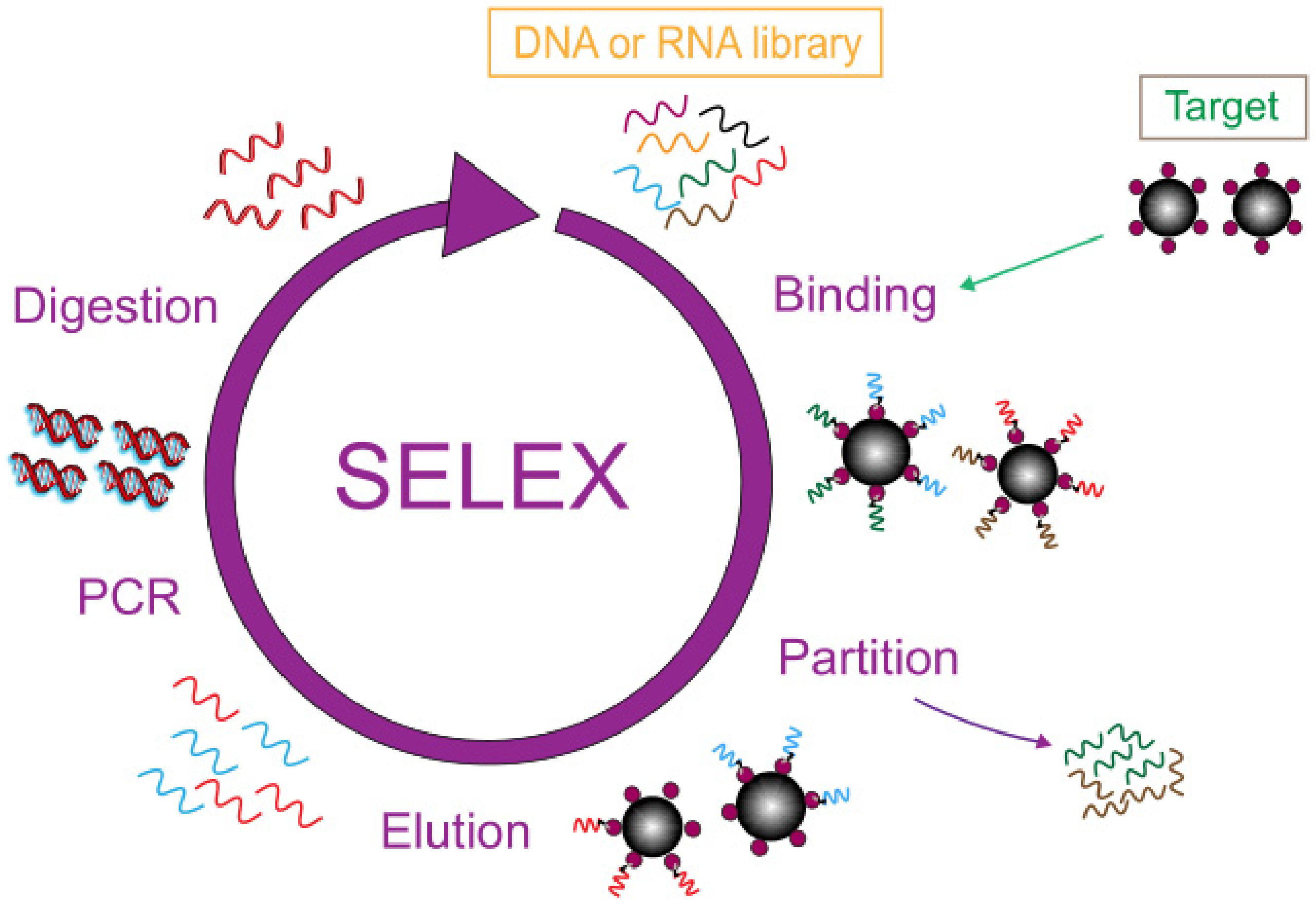
2. Application of Nanomaterials in Aptamer-Based Electrochemical Sensors
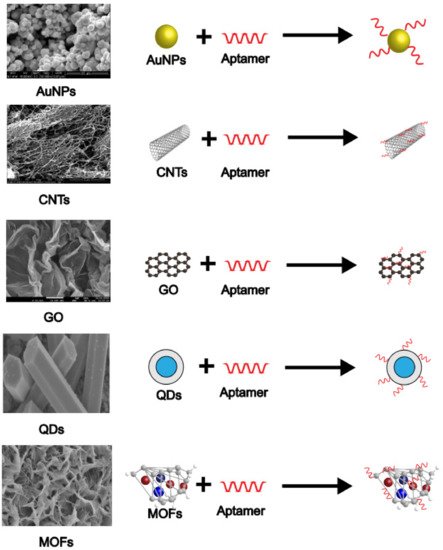
2.1. Gold Nanoparticles
2.2. Carbon Nanotubes
2.3. Graphene
2.4. Quantum Dots
2.5. Metal-Organic Frameworks
This entry is adapted from the peer-reviewed paper 10.3390/foods11101404
References
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Efremova Aaron, S.; Aaron, J.-J. Toxic Heavy Metals: Impact on the Environment and Human Health, and Treatment with Conducting Organic Polymers, a Review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942.
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy Metal Contamination Status in Greek Surface Waters: A Review with Application and Evaluation of Pollution Indices. Chemosphere 2021, 263, 128192.
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595.
- Fakhri, Y.; Saha, N.; Miri, A.; Baghaei, M.; Roomiani, L.; Ghaderpoori, M.; Taghavi, M.; Keramati, H.; Bahmani, Z.; Moradi, B.; et al. Metal Concentrations in Fillet and Gill of Parrotfish (Scarus Ghobban) from the Persian Gulf and Implications for Human Health. Food Chem. Toxicol. 2018, 118, 348–354.
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458.
- Al Hamouz, O.C.S.; Akintola, O.S. Removal of Lead and Arsenic Ions by a New Series of Aniline Based Polyamines. Process Saf. Environ. Prot. 2017, 106, 180–190.
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521.
- Harrington, C.F.; Clough, R.; Drennan-Harris, L.R.; Hill, S.J.; Tyson, J.F. Atomic Spectrometry Update. Elemental Speciation. J. Anal. At. Spectrom. 2011, 26, 1561.
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent Advances in Aptamer-Based Optical and Electrochemical Biosensors for Detection of Pesticides and Veterinary Drugs. Food Control 2022, 131, 108399.
- Li, F.; Yu, Z.; Han, X.; Lai, R.Y. Electrochemical Aptamer-Based Sensors for Food and Water Analysis: A Review. Anal. Chim. Acta 2019, 1051, 1–23.
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three Decades of Nucleic Acid Aptamer Technologies: Lessons Learned, Progress and Opportunities on Aptamer Development. Biotechnol. Adv. 2019, 37, 28–50.
- Zhu, G.; Chen, X. Aptamer-Based Targeted Therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78.
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519.
- Wang, T.; Chen, L.; Chikkanna, A.; Chen, S.; Brusius, I.; Sbuh, N.; Veedu, R.N. Development of Nucleic Acid Aptamer-Based Lateral Flow Assays: A Robust Platform for Cost-Effective Point-of-Care Diagnosis. Theranostics 2021, 11, 5174–5196.
- Wu, S.; Zhang, H.; Shi, Z.; Duan, N.; Fang, C.; Dai, S.; Wang, Z. Aptamer-Based Fluorescence Biosensor for Chloramphenicol Determination Using Upconversion Nanoparticles. Food Control 2015, 50, 597–604.
- Emrani, A.S.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric and Fluorescence Quenching Aptasensors for Detection of Streptomycin in Blood Serum and Milk Based on Double-Stranded DNA and Gold Nanoparticles. Food Chem. 2016, 190, 115–121.
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A Highly Sensitive Detection of Carbendazim Pesticide in Food Based on the Upconversion-MnO2 Luminescent Resonance Energy Transfer Biosensor. Food Chem. 2021, 349, 129157.
- You, H.; Bai, L.; Yuan, Y.; Zhou, J.; Bai, Y.; Mu, Z. An Amperometric Aptasensor for Ultrasensitive Detection of Sulfadimethoxine Based on Exonuclease-Assisted Target Recycling and New Signal Tracer for Amplification. Biosens. Bioelectron. 2018, 117, 706–712.
- Zhou, C.; Zou, H.; Sun, C.; Ren, D.; Xiong, W.; Li, Y. Fluorescent Aptasensor for Detection of Four Tetracycline Veterinary Drugs in Milk Based on Catalytic Hairpin Assembly Reaction and Displacement of G-Quadruplex. Anal. Bioanal. Chem. 2018, 410, 2981–2989.
- Chen, Q.; Sheng, R.; Wang, P.; Ouyang, Q.; Wang, A.; Ali, S.; Zareef, M.; Hassan, M.M. Ultra-Sensitive Detection of Malathion Residues Using FRET-Based Upconversion Fluorescence Sensor in Food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118654.
- Dolati, S.; Ramezani, M.; Nabavinia, M.S.; Soheili, V.; Abnous, K.; Taghdisi, S.M. Selection of Specific Aptamer against Enrofloxacin and Fabrication of Graphene Oxide Based Label-Free Fluorescent Assay. Anal. Biochem. 2018, 549, 124–129.
- Zhang, S.; Geryak, R.; Geldmeier, J.; Kim, S.; Tsukruk, V.V. Synthesis, Assembly, and Applications of Hybrid Nanostructures for Biosensing. Chem. Rev. 2017, 117, 12942–13038.
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2018, 7, 1700889.
- Baig, N.; Sajid, M.; Saleh, T.A. Recent Trends in Nanomaterial-Modified Electrodes for Electroanalytical Applications. TrAC Trends Anal. Chem. 2019, 111, 47–61.
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor Based on Fluorophore-Quencher Nano-Pair and Smartphone Spectrum Reader for on-Site Quantification of Multi-Pesticides. Biosens. Bioelectron. 2018, 117, 75–83.
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Hashemi Fath, R. Impedimetric Ultrasensitive Detection of Chloramphenicol Based on Aptamer MIP Using a Glassy Carbon Electrode Modified by 3-Ampy-RGO and Silver Nanoparticle. Colloids Surf. B Biointerfaces 2019, 183, 110451.
- Charbgoo, F.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Nanoparticles Application in High Sensitive Aptasensor Design. TrAC Trends Anal. Chem. 2016, 85, 85–97.
- Fernandes, P.M.V.; Campiña, J.M.; Silva, A.F. A Layered Nanocomposite of Laccase, Chitosan, and Fe3O4 Nanoparticles-Reduced Graphene Oxide for the Nanomolar Electrochemical Detection of Bisphenol A. Microchim. Acta 2020, 187, 262.
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55.
- Priyadarshini, E.; Pradhan, N. Gold Nanoparticles as Efficient Sensors in Colorimetric Detection of Toxic Metal Ions: A Review. Sens. Actuators B Chem. 2017, 238, 888–902.
- Yuan, M.; Qian, S.; Cao, H.; Yu, J.; Ye, T.; Wu, X.; Chen, L.; Xu, F. An Ultra-Sensitive Electrochemical Aptasensor for Simultaneous Quantitative Detection of Pb2+ and Cd2+ in Fruit and Vegetable. Food Chem. 2022, 382, 132173.
- Deng, W.; Hong, L.-R.; Zhao, M.; Zhuo, Y.; Gao, M. Electrochemiluminescence-Based Detection Method of Lead(ii) Ion via Dual Enhancement of Intermolecular and Intramolecular Co-Reaction. Analyst 2015, 140, 4206–4211.
- Liu, Y.; Lai, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S.; Wang, Z. Cd-Aptamer Electrochemical Biosensor Based on AuNPs/CS Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259.
- Yadav, R.; Kushwah, V.; Gaur, M.S.; Bhadauria, S.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Electrochemical Aptamer Biosensor for As 3+ Based on Apta Deep Trapped Ag-Au Alloy Nanoparticles-Impregnated Glassy Carbon Electrode. Int. J. Environ. Anal. Chem. 2020, 100, 623–634.
- Zhao, Y.; Xie, X. A Novel Electrochemical Aptamer Biosensor Based on DNAzyme Decorated Core-Shell Nanoparticles for Hg2+ Determination. J. Braz. Chem. Soc. 2018, 29, 232–239.
- Miao, P.; Tang, Y.; Wang, L. DNA Modified Fe3O4 @Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947.
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800.
- Charbgoo, F.; Behmanesh, M.; Nikkhah, M. Enhanced Reduction of Single-Wall Carbon Nanotube Cytotoxicity in Vitro: Applying a Novel Method of Arginine Functionalization. Biotechnol. Appl. Biochem. 2015, 62, 598–605.
- Wilder, J.W.G.; Venema, L.C.; Rinzler, A.G.; Smalley, R.E.; Dekker, C. Electronic Structure of Atomically Resolved Carbon Nanotubes. Nature 1998, 391, 59–62.
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc. 2005, 127, 11906–11907.
- Zhu, Y.; Zeng, G.; Zhang, Y.; Tang, L.; Chen, J.; Cheng, M.; Zhang, L.; He, L.; Guo, Y.; He, X.; et al. Highly Sensitive Electrochemical Sensor Using a MWCNTs/GNPs-Modified Electrode for Lead (II) Detection Based on Pb 2+ -Induced G-Rich DNA Conformation. Analyst 2014, 139, 5014.
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194.
- Rabai, S.; Teniou, A.; Catanante, G.; Benounis, M.; Marty, J.-L.; Rhouati, A. Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water. Sensors 2021, 22, 105.
- He, L.; Zhang, S.; Wang, M.; Peng, D.; Yan, F.; Zhang, Z.; Zhou, L. Facile Fabrication of Zinc Phosphate-Based Nanocomposites for High-Performance Electrochemical Sensing of Hg(II). Sens. Actuators B Chem. 2016, 228, 500–508.
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600.
- Biswas, C.; Lee, Y.H. Graphene Versus Carbon Nanotubes in Electronic Devices. Adv. Funct. Mater. 2011, 21, 3806–3826.
- Zhang, Y.; Xie, J.; Liu, Y.; Pang, P.; Feng, L.; Wang, H.; Wu, Z.; Yang, W. Simple and Signal-off Electrochemical Biosensor for Mercury(II) Based on Thymine-Mercury-Thymine Hybridization Directly on Graphene. Electrochim. Acta 2015, 170, 210–217.
- Hai, H.; Yang, F.; Li, J. Highly Sensitive Electrochemiluminescence “Turn-on” Aptamer Sensor for Lead(II) Ion Based on the Formation of a G-Quadruplex on a Graphene and Gold Nanoparticles Modified Electrode. Microchim. Acta 2014, 181, 893–901.
- Jiang, D.; Du, X.; Chen, D.; Zhou, L.; Chen, W.; Li, Y.; Hao, N.; Qian, J.; Liu, Q.; Wang, K. One-Pot Hydrothermal Route to Fabricate Nitrogen Doped Graphene/Ag-TiO2: Efficient Charge Separation, and High-Performance “on-off-on” Switch System Based Photoelectrochemical Biosensing. Biosens. Bioelectron. 2016, 83, 149–155.
- Wang, L.; Peng, X.; Fu, H. An Electrochemical Aptasensor for the Sensitive Detection of Pb2+ Based on a Chitosan/Reduced Graphene Oxide/Titanium Dioxide. Microchem. J. 2022, 174, 106977.
- Li, H.; Xue, Y.; Wang, W. Femtomole Level Photoelectrochemical Aptasensing for Mercury Ions Using Quercetin–Copper(II) Complex as the DNA Intercalator. Biosens. Bioelectron. 2014, 54, 317–322.
- Esteve-Turrillas, F.A.; Abad-Fuentes, A. Applications of Quantum Dots as Probes in Immunosensing of Small-Sized Analytes. Biosens. Bioelectron. 2013, 41, 12–29.
- Shahdost-fard, F.; Roushani, M. Designing an Ultra-Sensitive Aptasensor Based on an AgNPs/Thiol-GQD Nanocomposite for TNT Detection at Femtomolar Levels Using the Electrochemical Oxidation of Rutin as a Redox Probe. Biosens. Bioelectron. 2017, 87, 724–731.
- Khonsari, Y.N.; Sun, S. A Novel Label Free Electrochemiluminescent Aptasensor for the Detection of Lysozyme. Mater. Sci. Eng. C 2019, 96, 146–152.
- Cao, J.-T.; Liao, X.-J.; Wang, Y.-L.; Liu, Y.-M. A Novel Photoelectrochemical Strategy for Lead Ion Detection Based on CdSe Quantum Dots Co-Sensitized ZnO-CdS Nanostructure. J. Electroanal. Chem. 2021, 880, 114828.
- Adegoke, O.; Daeid, N.N. Alloyed AuFeZnSe Quantum Nanorod Nanocomposite as an Ultrasensitive and Selective Plasmon-Amplified Fluorescence OFF-ON Aptasensor for Arsenic (III). J. Photochem. Photobiol. A Chem. 2022, 426, 113755.
- Shi, J.-J.; Zhu, J.-C.; Zhao, M.; Wang, Y.; Yang, P.; He, J. Ultrasensitive Photoelectrochemical Aptasensor for Lead Ion Detection Based on Sensitization Effect of CdTe QDs on MoS2-CdS:Mn Nanocomposites by the Formation of G-Quadruplex Structure. Talanta 2018, 183, 237–244.
- Feng, D.; Li, P.; Tan, X.; Wu, Y.; Wei, F.; Du, F.; Ai, C.; Luo, Y.; Chen, Q.; Han, H. Electrochemiluminescence Aptasensor for Multiple Determination of Hg2+ and Pb2+ Ions by Using the MIL-53(Al)@CdTe-PEI Modified Electrode. Anal. Chim. Acta 2020, 1100, 232–239.
- Li, L.; Chen, B.; Luo, L.; Liu, X.; Bi, X.; You, T. Sensitive and Selective Detection of Hg2+ in Tap and Canal Water via Self-Enhanced ECL Aptasensor Based on NH2– Talanta 2021, 222, 121579.
- Zang, Y.; Lei, J.; Hao, Q.; Ju, H. “Signal-On” Photoelectrochemical Sensing Strategy Based on Target-Dependent Aptamer Conformational Conversion for Selective Detection of Lead(II) Ion. ACS Appl. Mater. Interfaces 2014, 6, 15991–15997.
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2021, 31, 2006970.
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-Functionalized Metal-Organic Frameworks (MOFs) for Biosensing. Biosens. Bioelectron. 2021, 176, 112947.
- Pavadai, R.; Amalraj, A.; Subramanian, S.; Perumal, P. High Catalytic Activity of Fluorophore-Labeled Y-Shaped DNAzyme/3D MOF-MoS 2 NBs as a Versatile Biosensing Platform for the Simultaneous Detection of Hg 2+, Ni 2+, and Ag + Ions. ACS Appl. Mater. Interfaces 2021, 13, 31710–31724.
- Zhang, Z.-H.; Duan, F.-H.; Tian, J.-Y.; He, J.-Y.; Yang, L.-Y.; Zhao, H.; Zhang, S.; Liu, C.-S.; He, L.-H.; Chen, M.; et al. Aptamer-Embedded Zirconium-Based Metal–Organic Framework Composites Prepared by De Novo Bio-Inspired Approach with Enhanced Biosensing for Detecting Trace Analytes. ACS Sens. 2017, 2, 982–989.
- Ling, P.; Lei, J.; Ju, H. Porphyrinic Metal-Organic Framework as Electrochemical Probe for DNA Sensing via Triple-Helix Molecular Switch. Biosens. Bioelectron. 2015, 71, 373–379.
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.-Y.; He, L.; Zhang, X.; Liu, C.-S. Fe(III)-Based Metal–Organic Framework-Derived Core–Shell Nanostructure: Sensitive Electrochemical Platform for High Trace Determination of Heavy Metal Ions. Biosens. Bioelectron. 2017, 94, 358–364.
