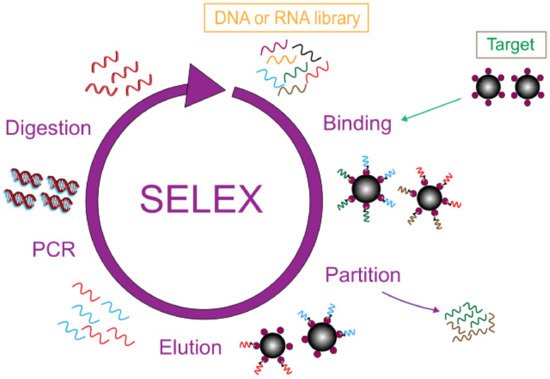
2. Application of Nanomaterials in Aptamer-Based Electrochemical Sensors
In recent years, the successful synthesis of various nanomaterials has attracted significant attention. Their unique physical and chemical properties, including high surface area/volume ratio, high reactivity, size dependence, and high functionalization, make them widely used in chemical analysis, therapy, diagnosis, and food safety [
20,
21,
22,
23,
24]. In addition to the binding activity of the aptamer, the reason why the electrochemical aptamer sensors can detect heavy metal ions as low as fM is largely attributed to the rational use of nanomaterials. Sensing platforms are formed by immobilizing aptamers on nanomaterial surfaces through intermolecular forces or catalyzing chemical reactions in sensors as sensitizers to enhance electrical signals and improve the specificity and sensitivity of sensors [
25]. Due to their recognized properties, the coupling of aptamers on nanomaterials has great potential to form biosensor platforms.
Gold nanoparticles, carbon nanotubes, graphene, quantum dots, and metal-organic frameworks are common and basic nanomaterials used in E-apt sensors. These materials can be mixed or coupled with other substances to create new composite materials. They have shown many advantages such as high specific surface area, good biocompatibility, high electrical conductivity, high magnetic properties, and unique electro-optic and physicochemical properties [
26,
27,
28]. In addition to being used as sensitizers, most nanomaterials in E-apt sensors are used to fix aptamers to form couplings with a better capture effect. Researchers often use couplings as probes to obtain better detection results.
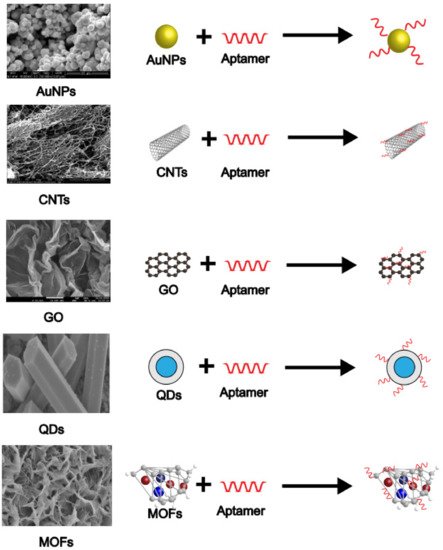
Figure 4 indicates how popular nanomaterials (shown as their microscopic morphology) are coupled with aptamers.
Figure 4. SEM images and combination modes of various nanomaterials.
2.1. Gold Nanoparticles
Among the aptamer-based sensors developed, metal nanoparticles such as gold nanoparticles (AuNPs) are the most common advanced materials. They have high stability and oxidation resistance and can be prepared through various physical and chemical methods, among which the most classical preparation method was proposed by Turkevich et al. in 1951 [
29]. The high surface-volume ratio and excellent optical properties of AuNPs contribute to the high sensitivity and selectivity of the target detection. The optical properties of AuNPs are largely dependent on the size, shape, and aggregation state of nanoparticles. Colloidal AuNPs are usually red or pink and turn purplish-blue when aggregated, forming the basis of colorimetric detection [
30]. AuNPs are also conductive and fluorescent and, thus, have the ability to respond to external electrochemical and optical stimulation.
One of the most common approaches to using AuNPs in electrochemical sensors is to anchor aptamers. The aptamer is usually fixed by the force of the Au-S bond; Yuan et al. [
31] used this method to develop a sensor for detecting Pb
2+ and Cd
2+ at the same time. Due to the specific binding of aptamers to metal ions, methylene blue or ferrocene labeled aptamers were competitively separated from the gold electrode, resulting in a weaker electrochemical signal. The detection limits of Cd
2+ and Pb
2+ were 89.31 and 16.44 pM. Deng et al. [
32] deposited L-cysteine and AuNPs layers on the electrode surface successively providing a large surface area to anchor many sulfur-capped auxiliary probes through the mercaptan-gold interaction. Additionally, to fix the aptamer, Liu et al. used chitosan to attach AuNPs to the electrode to enhance electron transfer, thus improving the sensitivity of the sensor [
33].
In addition to being used alone, AuNPs are also used as composite materials coupled with other metal ions. The composite materials tend to enhance the electrochemical signal so that even subtle changes can be detected. Silver and gold alloy nanoparticles (Ag-Au alloy NPs) promise to create inexpensive and stable electrochemical sensors [
34]. This is because silver-gold alloy nanoparticles have a large specific surface area and good electrical conductivity which can act as a conductive center and promote electron transfer, thus trapping more heavy metal ions on the electrode. Zhao et al. [
35] used DNA enzyme functionalized (Ag-Au alloy) NPs to form core-shell nanoparticles, which can be used as signal tags. Because the core-shell structure increases the specific surface area and catalyzes the reaction, the electrochemical signal can be enhanced. Ag-Au Alloy NPs were also used to modify GCE and the resulting sensor had higher sensitivity and reproducibility. Miao et al. [
36] used Fe
3O
4@AuNPs to carry a DNA probe, and the other two were labeled with independent electrochemical substances which can detect both Hg
2+ and Ag
2+. Due to their excellent signal amplification, AuNPs have been one of the most used transduction materials to construct E-apt sensors for the analysis of food and water contaminants.
2.2. Carbon Nanotubes
Carbon nanotubes (CNTs) are a kind of carbon nanomaterial with unique electrical transmission properties and a large specific surface area, with excellent chemical, mechanical, and thermal stability, and direct binding with other molecules. Therefore, they have received great attention in biosensor design [
37,
38]. They can be thought of as cylindrical tubes made of one or more sheets of graphene rolled and folded. Nanotubes formed from a single sheet of graphene are called single-walled nanotubes (SWNT), with diameters between 0.4~2 nm, and multi-walled carbon nanotubes(MWCNTs) are 2~10 times larger [
39]. Graphene contains SP
2 hybrid carbon atoms, and nucleic acids can easily be attached to the surface of the nanotubes through π-π bonds, so CNTs are often used to carry ssDNA or RNA. In 2005, So et al. [
40] constructed a biosensor using single-walled CNTs by using aptamer as a molecular recognition element for the first time. Since then, the potential of CNTs in sensing platforms has been gradually explored.
It is more common to modify electrodes with MWCNTs and then fix aptamers on the surface of MWCNTs. Zhu et al. [
41] used carboxylic acid group functionalized MWCNTs to fix aptamers. Because the material has a large surface area and good charge transferability, the DNA attachment amount and sensor performance could be significantly improved. The sensor could detect Pb
2+ in the range of 5.0 × 10
−11~1.0 × 10
−14 M. Additionally, Zou et al. [
42] covalently immobilized aptamers on carboxylic functionalized MWCNTs by EDC/NHS chemistry. CNTs can not only carry aptamers in sensors but also provide binding sites for the reactions between aptamers and other nanomaterials. Rabai et al. [
43] modified the electrode surface with CNTs, then deposited AuNPs on the composite electrode. Finally, with the aptamer fixed, the sensor had a detection limit of 0.02 pM for Cd
2+. He et al. [
44] developed an electrochemical sensor with a Zn
3(PO
4)
2 modified aptamer and found that the sensor with MWCNTs had higher sensitivity.
2.3. Graphene
Graphene is a two-dimensional carbon nanomaterial with SP
2 hybrid connected carbon atoms tightly packed into a single layer honeycomb lattice structure. Due to its excellent electrical properties, graphene has attracted great interest in recent years. Graphene has a unique type of structure that also gives it features not found in many other nanomaterials; its large specific surface area makes it an ideal candidate for immobilizing large numbers of functionalized metal oxides and noble metal nanoparticles [
45]. Exceptional carrier mobility offers great prospects for nanoscale applications, such as electronic devices and chemical/biological sensors. In addition, its high electron transfer efficiency and wide electrochemical window make graphene an ideal material for constructing a highly sensitive E-apt sensor [
46]. In recent years, graphene as a REDOX molecule in electrochemical sensors has attracted great attention.
The most common use of graphene in sensors is to modify electrodes. Zhang et al. [
47] developed an Hg
2+ detector for a graphene-fixed aptamer probe with a detection limit of 5 pM. Using graphene-modified electrodes, the modified electrode surface can be reused. Similar to AuNPs, it is more likely that graphene will be coupled with other nanomaterials to form new composite materials for better modification. Hai et al. [
48] modified the electrode with AuNPs coupled with graphene. The aptamer was self-assembled on the electrode, and the detection limit of Pb
2+ was as low as 3.8 pM. Jiang et al. [
49] designed a new nanometer composite material consisting of TiO
2, AuNPs, and nitrogen-doped graphene. The composite exhibits excellent optical properties, increasing the exciton lifetime and improving the charge transfer photocurrent intensity to 18.2 times higher than that of original TiO
2. Wang et al. [
50] synthesized another new material modified electrode by using CS instead of AuNPs for the detection of Pb
2+. The modified electrode showed good repeatability, stability, and specificity to other interfering metal ions. Li et al. [
51] prepared a sensor for mercury ions based on a perylene-3, 4, 9, 10-tetracarboxylic acid/graphene oxide (PTCA/GO) and quercetin-copper(II) complex. They efficiently promoted the separation of photoexcited carriers and enhanced the photocurrent.
2.4. Quantum Dots
Quantum dots (QDs), also known as semiconductor nanocrystals, are mixtures of cadmium and selenium or tellurium with nanoscale clusters with diameters of 1–20 nm [
52]. Due to their unique properties, such as high quantum yield, excitation-dependent emission, surface modification versatility, and long-term photostability [
53,
54], QDs have been used in many research fields, especially in nanoelectronics, optoelectronics, and biological analysis [
55]. The physical size of the nanocrystals determines the wavelength of the emitted fluorescence, so multiple analyses can be performed using a single excitation source. With the rapid development of nanotechnology, such nanomaterials have been widely used to improve the sensitivity of sensors. When they are incorporated into the design of biosensors, they can be used as tags, as part of a signal sensor [
56].
QDs are mainly used in electrochemical luminescence sensors and photoelectric electrochemistry sensors. When the aptamer reacts with the corresponding target, the brightness of the QDs will also change due to the resonance energy transfer, thus improving the sensitivity of the sensor. CdTe QDs are most frequently used in sensors; Shi et al. [
57] developed a new method for the detection of Pb
2+ based on the sensitization effect of CdTe QDs. When the target is present, the labeled QDs close to the electrode surface produce a sensitization effect and the photocurrent intensity is enhanced. Feng et al. [
58] modified CdTe QDs with MIL-53 and determined Hg
2+ and Pb
2+ simultaneously by the ECL method with good recovery. Except for CdTe QDs, there are many other types of QDs, such as CdS and nitrogen-doped graphene (NG) [
59,
60], and their principle of action is roughly the same. When the conformation of the aptamer changes, the distance between the QDs and the electrode alters, and the electrical signal is either enhanced or weakened. This property makes QDs widely used to design E-apt sensors for food and water analysis.
2.5. Metal-Organic Frameworks
In recent years, more and more metal-organic frameworks (MOFs) have been found to effectively coordinate polymer nanomaterials and have received extensive attention as effective quenching materials. MOFs are a kind of crystalline nanomaterial composed of metal ions and organic ligands. Due to their water dispersibility, adjustability, biocompatibility, low cost, controllable shape, ultra-high porosity, and high specific surface area [
61], MOFs have been increasingly used in biosensors, electrocatalysis, energy storage, and conversion. The large specific surface area and ultra-high porosity of MOFs provide more reaction sites for aptamers and targets. Organic ligands with rich functional groups make MOFs easy to be functionalized with various molecules and materials [
62]. In addition, MOF compositions take a variety of forms (e.g., nanosheets, cages, tubes, rods, cubes, etc.) and can be easily adjusted according to the selection of various organic connectives and metal ions [
63].
Aptamers are securely fixed in the MOFs by encapsulation. The main framework of MOFs can facilitate various interactions with analytes through functional groups in organic ligands, thus achieving high sensitivity and high selectivity recognition. Therefore, MOFs can be used as signal probes for different detection methods. Zhang et al. [
64] used a Zr-based MOF embedded with three kinds of aptamer. Ling et al. [
65] prepared streptavidin functionalized zirconium porphyrin MOF (PCN-222@SA) using a covalent method as a signal nanoprobe. Introducing this signal nanoprobe into the sensor surface significantly amplified the electrocatalytic current. Zhang et al. [
66] synthesized a core-shell nanostructured composite material composed of Fe (III)-based MOF (Fe-MOF) and mesoporous Fe
3O
4@C nanocapsules (Fe-MOF@mFe
3O
4 @MC) that exhibited excellent electrochemical activity, water stability, and high specific surface area, resulting in strong biological binding to heavy metal ion targeting aptamer chains.