
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ruili Yin | -- | 2071 | 2022-05-25 02:37:58 | | | |
| 2 | Peter Tang | Meta information modification | 2071 | 2022-05-25 03:34:58 | | |
Video Upload Options
Dipeptidyl peptidase 4 (DPP4) enzyme is a type II transmembrane glycoprotein, expressed ubiquitously in many tissues, including the immune cells, kidney, liver, pancreas, fat cells, and presents as a soluble form in the circulation. Dipeptidyl peptidase 4 is a serine protease, can cleave and inactivate incretin hormones, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), neuropeptides, and chemokines. In addition, DPP4 has been shown to have a direct pro-inflammatory role in lymphocytes, macrophages, and smooth muscle cell.
1. Introduction
2. DPP4 and DPP4 Inhibitions in Diabetes
2.1. Mechanisms of Effect of DPP4i
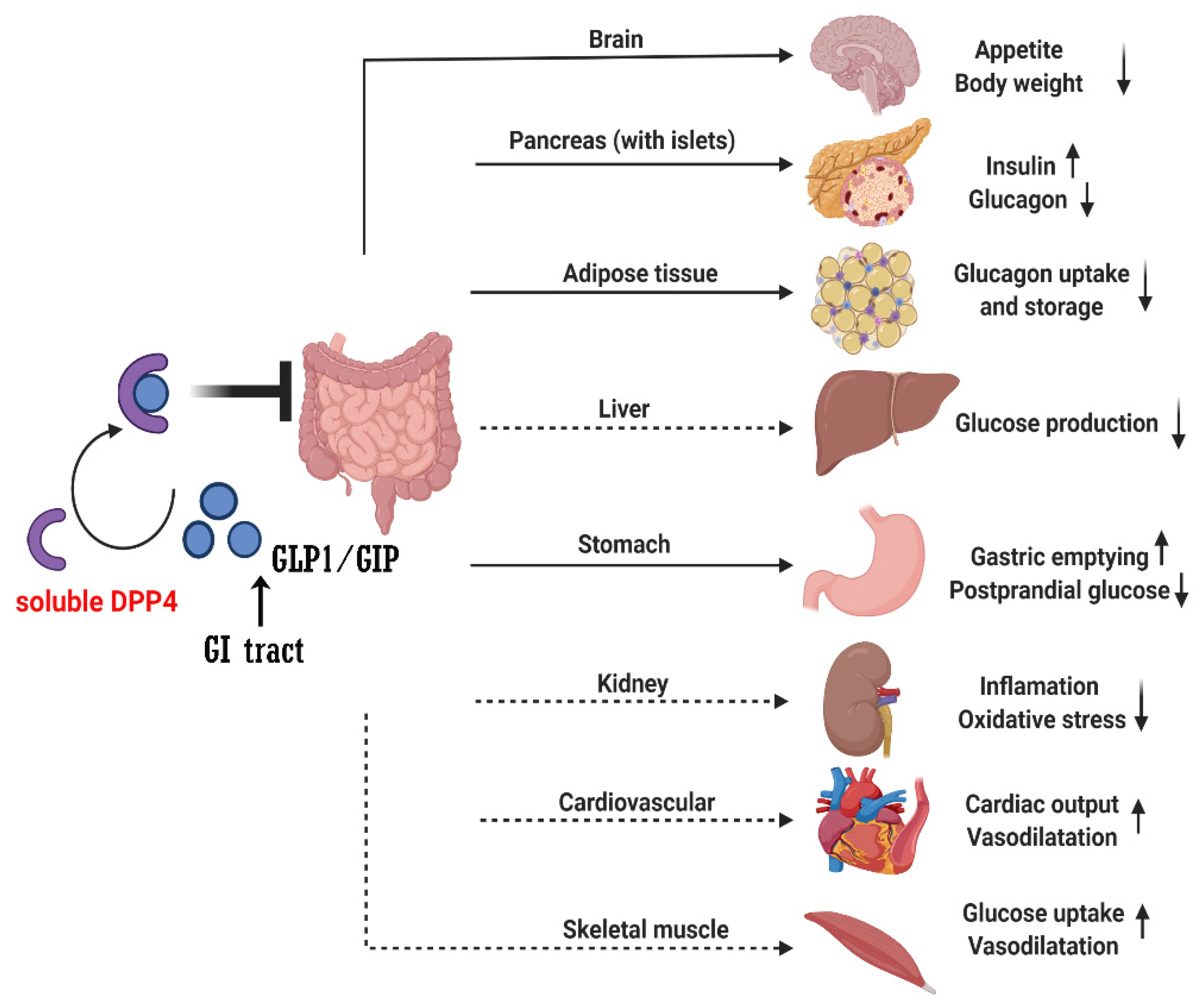
2.2. DPP4 Inhibitors
|
DPP4i |
Chemistry |
Metabolism |
Half-Life |
Elimination Method |
|---|---|---|---|---|
|
Sitagliptin |
β-amino acid based |
Minimal |
12.5 h |
Predominantly (>80%) renal |
|
Vildagliptin |
Cyanopyrrolidine |
Hydrolysis (cytochromeindependent) to form an inactive metabolite |
~2 h |
Metabolism (parent) and renal (metabolite) |
|
Saxagliptin |
Cyanopyrrolidine |
Hydrolysis (cytochrome P450 3A4 or P450 3A5) to form an activemetabolite |
2.5 h (parent), 3 h (metabolite) |
Metabolism (parent) and renal (metabolite) |
|
Alogliptin |
Modified pyrimidinedione |
Minimal |
20 h |
Predominantly (>70%) renal |
|
Linagliptin |
Xanthine based |
Minimal |
~12 h (effective), >100 h (terminal) |
Predominantly biliary (<6% renal) |
2.3. Benefits of DPP4i
|
DPP4i |
Trial (Year) |
Median Follow-Up, Years |
Mean/Median Age, Years |
Female (Total) |
BMI, kg/m2 * |
HbA1c, mmol/mol (%) * |
Baseline Metformin, % |
Baseline eGFR, mL/min/ [1.73 m]2 * |
Prior ASCVD, % |
Prior CHF, % |
|---|---|---|---|---|---|---|---|---|---|---|
|
Sitagliptin |
TECOS (2015) |
3.0 |
65 |
4212 |
30.2 |
55 (7.2) |
81 |
75 |
100 |
18 |
|
(14,523) |
||||||||||
|
Saxagliptin |
SAVOR-TIMI (2013) |
2.1 |
65 |
5590 |
31.2 |
64 (8.0) |
69 |
73 |
78 |
13 |
|
(16,492) |
||||||||||
|
Alogliptin |
EXAMINE (2013) |
1.5 |
61 |
1722 |
28.7 |
64 (8.0) |
NA |
71 |
100 |
28 |
|
(5380) |
||||||||||
|
Linagliptin |
CARMEL (2019) |
2.2 |
66 |
2582 |
31.4 |
64 (8.0) |
54 |
55 |
57 |
27 |
NA, not available; * These are expressed as mean values.
|
DPP4i |
Dose (mg/Day) |
HbA1c Reduction |
|---|---|---|
|
Sitagliptin |
100 |
0.5–1.0 |
|
Saxagliptin |
5 |
0.5–1.0 |
|
Alogliptin |
25 |
0.6 (mean value) |
|
Linagliptin |
5 |
0.5–0.7 |
2.4. Anti-Inflammation Effects of DPP4i
2.5. Adverse Effects
References
- Kos, K.; Baker, A.R.; Jernas, M.; Harte, A.L.; Clapham, J.C.; O’Hare, J.P.; Carlsson, L.; Kumar, S.; McTernan, P.G. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes. Metab. 2009, 11, 285–292.
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24.
- Wronkowitz, N.; Gorgens, S.W.; Romacho, T.; Villalobos, L.A.; Sanchez-Ferrer, C.F.; Peiro, C.; Sell, H.; Eckel, J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim. Biophys. Acta 2014, 1842, 1613–1621.
- Ghorpade, D.S.; Ozcan, L.; Zheng, Z.; Nicoloro, S.M.; Shen, Y.; Chen, E.; Bluher, M.; Czech, M.P.; Tabas, I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 2018, 555, 673–677.
- Holst, J.J.; Deacon, C.F. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes 1998, 47, 1663–1670.
- Ahren, B. Dipeptidyl peptidase-4 inhibitors: Clinical data and clinical implications. Diabetes Care 2007, 30, 1344–1350.
- Fan, L.; Zhou, W.; Zhang, L.; Jiang, D.; Zhao, Q.; Liu, L. Sitagliptin protects against hypoxia/reoxygenation (H/R)-induced cardiac microvascular endothelial cell injury. Am. J. Transl. Res. 2019, 11, 2099–2107.
- Kirino, Y.; Sato, Y.; Kamimoto, T.; Kawazoe, K.; Minakuchi, K.; Nakahori, Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: A streptozotocin-induced model using wild-type and DPP4-deficient rats. J. Endocrinol. 2009, 200, 53–61.
- Kirino, Y.; Sato, Y.; Kamimoto, T.; Kawazoe, K.; Minakuchi, K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E372–E379.
- Wang, X.; Xiang, J.; Huang, G.; Kang, L.; Yang, G.; Wu, H.; Jiang, K.; Liang, Z.; Yang, S. Inhibition of Podocytes DPP4 Activity Is a Potential Mechanism of Lobeliae Chinensis Herba in Treating Diabetic Kidney Disease. Front. Pharmacol. 2021, 12, 779652.
- Zheng, T.P.; Liu, Y.H.; Yang, L.X.; Qin, S.H.; Liu, H.B. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of subclinical atherosclerosis in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Atherosclerosis 2015, 242, 580–588.
- Varin, E.M.; Mulvihill, E.E.; Beaudry, J.L.; Pujadas, G.; Fuchs, S.; Tanti, J.F.; Fazio, S.; Kaur, K.; Cao, X.; Baggio, L.L.; et al. Circulating Levels of Soluble Dipeptidyl Peptidase-4 Are Dissociated from Inflammation and Induced by Enzymatic DPP4 Inhibition. Cell Metab. 2019, 29, 320–334.e5.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414.
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236.
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744.
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80.
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331.
- Goossens, G.H.; Blaak, E.E. Adipose tissue dysfunction and impaired metabolic health in human obesity: A matter of oxygen? Front. Endocrinol. 2015, 6, 55.
- Muller, T.D.; Bluher, M.; Tschop, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223.
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842.
- Rask-Andersen, M.; Almen, M.S.; Schioth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590.
- Kaur, P.; Mittal, A.; Nayak, S.K.; Vyas, M.; Mishra, V.; Khatik, G.L. Current Strategies and Drug Targets in the Management of Type 2 Diabetes Mellitus. Curr. Drug Targets 2018, 19, 1738–1766.
- Deacon, C.F.; Hughes, T.E.; Holst, J.J. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes 1998, 47, 764–769.
- Xiang, X.; Lang, M.; Li, Y.; Zhao, X.; Sun, H.; Jiang, W.; Ni, L.; Song, Y. Purification, identification and molecular mechanism of dipeptidyl peptidase IV inhibitory peptides from discarded shrimp (Penaeus vannamei) head. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1186, 122990.
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019.
- Rohrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386.
- Ahren, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376.
- De, S.; Banerjee, S.; Kumar, S.K.A.; Paira, P. Critical Role of Dipeptidyl Peptidase IV: A Therapeutic Target for Diabetes and Cancer. Mini-Rev. Med. Chem. 2019, 19, 88–97.
- Shimizu, S.; Hosooka, T.; Matsuda, T.; Asahara, S.; Koyanagi-Kimura, M.; Kanno, A.; Bartolome, A.; Etoh, H.; Fuchita, M.; Teruyama, K.; et al. DPP4 inhibitor vildagliptin preserves beta-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J. Mol. Endocrinol. 2012, 49, 125–135.
- Jonik, S.; Marchel, M.; Grabowski, M.; Opolski, G.; Mazurek, T. Gastrointestinal Incretins-Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease-State of the Art. Biology 2022, 11, 288.
- Bekiari, E.; Rizava, C.; Athanasiadou, E.; Papatheodorou, K.; Liakos, A.; Karagiannis, T.; Mainou, M.; Rika, M.; Boura, P.; Tsapas, A. Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine 2016, 52, 458–480.
- Scott, L.J. Sitagliptin: A Review in Type 2 Diabetes. Drugs 2017, 77, 209–224.
- Aulinger, B.A.; Bedorf, A.; Kutscherauer, G.; de Heer, J.; Holst, J.J.; Goke, B.; Schirra, J. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes 2014, 63, 1079–1092.
- Nauck, M.A.; Kind, J.; Kothe, L.D.; Holst, J.J.; Deacon, C.F.; Broschag, M.; He, Y.L.; Kjems, L.; Foley, J. Quantification of the Contribution of GLP-1 to Mediating Insulinotropic Effects of DPP-4 Inhibition With Vildagliptin in Healthy Subjects and Patients With Type 2 Diabetes Using Exendin as a GLP-1 Receptor Antagonist. Diabetes 2016, 65, 2440–2447.
- Sharma, A.; Paliwal, G.; Upadhyay, N.; Tiwari, A. Retraction Note: Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J. Diabetes Metab. Disord. 2015, 15, 34.
- Sharma, A.; Paliwal, G.; Upadhyay, N.; Tiwari, A. Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J. Diabetes Metab. Disord. 2015, 14, 15.
- Vilsboll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 2002, 45, 1111–1119.
- Mentis, N.; Vardarli, I.; Kothe, L.D.; Holst, J.J.; Deacon, C.F.; Theodorakis, M.; Meier, J.J.; Nauck, M.A. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 2011, 60, 1270–1276.
- Hojberg, P.V.; Vilsboll, T.; Rabol, R.; Knop, F.K.; Bache, M.; Krarup, T.; Holst, J.J.; Madsbad, S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009, 52, 199–207.
- Aaboe, K.; Akram, S.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Krarup, T. Restoration of the insulinotropic effect of glucose-dependent insulinotropic polypeptide contributes to the antidiabetic effect of dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2015, 17, 74–81.
- Nakamura, T.; Tanimoto, H.; Okamoto, M.; Takeuchi, M.; Tsubamoto, Y.; Noda, H. GIP Receptor Antagonist, SKL-14959 Indicated Alteration of the Lipids Metabolism to Catabolism by the Inhibition of Plasma LPL Activity, Resulting in the Suppression of Weight Gain on Diets-Induced Obesity Mice. Diabetes Metab. Syndr. Obes. 2021, 14, 1095–1105.
- Gasbjerg, L.S.; Bari, E.J.; Stensen, S.; Hoe, B.; Lanng, A.R.; Mathiesen, D.S.; Christensen, M.B.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M.; et al. Dose-dependent efficacy of the glucose-dependent insulinotropic polypeptide (GIP) receptor antagonist GIP(3–30)NH2 on GIP actions in humans. Diabetes Obes. Metab. 2021, 23, 68–74.
- Christensen, M.B. Glucose-dependent insulinotropic polypeptide: Effects on insulin and glucagon secretion in humans. Dan. Med. J. 2016, 63, B5230.
- Ahren, B.; Schweizer, A.; Dejager, S.; Dunning, B.E.; Nilsson, P.M.; Persson, M.; Foley, J.E. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 1236–1243.
- Farngren, J.; Persson, M.; Schweizer, A.; Foley, J.E.; Ahren, B. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 812–818.
- Mannucci, E.; Nreu, B.; Montereggi, C.; Ragghianti, B.; Gallo, M.; Giaccari, A.; Monami, M.; SID-AMD Joint Panel for Italian Guidelines on Treatment of Type 2 Diabetes. Cardiovascular events and all-cause mortality in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2745–2755.
- Molina-Vega, M.; Munoz-Garach, A.; Fernandez-Garcia, J.C.; Tinahones, F.J. The safety of DPP-4 inhibitor and SGLT2 inhibitor combination therapies. Expert Opin. Drug Saf. 2018, 17, 815–824.
- Biftu, T.; Feng, D.; Qian, X.; Liang, G.B.; Kieczykowski, G.; Eiermann, G.; He, H.; Leiting, B.; Lyons, K.; Petrov, A.; et al. (3R)-4--3-(2,2,2-trifluoroethyl)-1,4-diazepan-2-one, a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. Lett. 2007, 17, 49–52.
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and preclinical profile of Saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 5025–5037.
- Choy, M.; Lam, S. Sitagliptin: A novel drug for the treatment of type 2 diabetes. Cardiol. Rev. 2007, 15, 264–271.
- Thareja, S.; Aggarwal, S.; Malla, P.; Haksar, D.; Bhardwaj, T.R.; Kumar, M. Saxagliptin: A new drug for the treatment of type 2 diabetes. Mini-Rev. Med. Chem. 2010, 10, 759–765.
- White, J.R. Alogliptin for the treatment of type 2 diabetes. Drugs Today 2011, 47, 99–107.
- Aletti, R.; Cheng-Lai, A. Linagliptin: The newest dipeptidyl peptidase-4 inhibitor for type 2 diabetes mellitus. Cardiol. Rev. 2012, 20, 45–51.
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79.
- Biessels, G.J.; Verhagen, C.; Janssen, J.; van den Berg, E.; Zinman, B.; Rosenstock, J.; George, J.T.; Passera, A.; Schnaidt, S.; Johansen, O.E.; et al. Effect of Linagliptin on Cognitive Performance in Patients With Type 2 Diabetes and Cardiorenal Comorbidities: The CARMELINA Randomized Trial. Diabetes Care 2019, 42, 1930–1938.
- Chikata, Y.; Iwata, H.; Miyosawa, K.; Koike, T.; Yasuda, H.; Funamizu, T.; Doi, S.; Endo, H.; Wada, H.; Naito, R.; et al. Dipeptidyl peptidase-4 inhibitors reduced long-term cardiovascular risk in diabetic patients after percutaneous coronary intervention via insulin-like growth factor-1 axis. Sci. Rep. 2022, 12, 5129.
- Carr, R.D.; Katzeff, H.L.; Alexander, C.M.; Berger, J.P.; Xu, S.S.; Thornberry, N. Reply to: Ahren, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E.; Foley, J.E. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes. Metab. 2011, 13, 775–783 and Ahren, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E.; Foley, J.E. Clinical evidence and mechanistic basis for vildagliptin’s action when added to metformin. Diabetes Obes. Metab. 2011, 13, 193–203, Diabetes Obes. Metab.2012, 14, 383–384.
- Nabeno, M.; Akahoshi, F.; Kishida, H.; Miyaguchi, I.; Tanaka, Y.; Ishii, S.; Kadowaki, T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Commun. 2013, 434, 191–196.
- Tatosian, D.A.; Guo, Y.; Schaeffer, A.K.; Gaibu, N.; Popa, S.; Stoch, A.; Langdon, R.B.; Kauh, E.A. Dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes treated with saxagliptin, sitagliptin, or vildagliptin. Diabetes Ther. 2013, 4, 431–442.
- Baranov, O.; Kahle, M.; Deacon, C.F.; Holst, J.J.; Nauck, M.A. Feedback suppression of meal-induced glucagon-like peptide-1 (GLP-1) secretion mediated through elevations in intact GLP-1 caused by dipeptidyl peptidase-4 inhibition: A randomized, prospective comparison of sitagliptin and vildagliptin treatment. Diabetes Obes. Metab. 2016, 18, 1100–1109.
- Alsalim, W.; Goransson, O.; Tura, A.; Pacini, G.; Mari, A.; Ahren, B. Persistent whole day meal effects of three dipeptidyl peptidase-4 inhibitors on glycaemia and hormonal responses in metformin-treated type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 590–598.
- Scheen, A.J.; Charpentier, G.; Ostgren, C.J.; Hellqvist, A.; Gause-Nilsson, I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2010, 26, 540–549.
- Addy, C.; Tatosian, D.; Glasgow, X.S.; Gendrano, I.N., 3rd; Kauh, E.; Martucci, A.; Johnson-Levonas, A.O.; Selverian, D.; Matthews, C.Z.; Gutierrez, M.; et al. Pharmacokinetic and Pharmacodynamic Effects of Multiple-dose Administration of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor, in Obese Participants With and Without Type 2 Diabetes Mellitus. Clin. Ther. 2016, 38, 516–530.
- Kim, Y.G.; Hahn, S.; Oh, T.J.; Kwak, S.H.; Park, K.S.; Cho, Y.M. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: A systematic review and meta-analysis. Diabetologia 2013, 56, 696–708.
- Cai, X.; Han, X.; Luo, Y.; Ji, L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on beta-cell function in Asian and Caucasian type 2 diabetes mellitus patients: A meta-analysis. J. Diabetes 2015, 7, 347–359.
- Gao, W.; Wang, Q.; Yu, S. Efficacy, safety and impact on beta-cell function of dipeptidyl peptidase-4 inhibitors plus metformin combination therapy in patients with type 2 diabetes and the difference between Asians and Caucasians: A meta-analysis. J. Endocrinol. Investig. 2016, 39, 1061–1074.
- Kozlovski, P.; Fonseca, M.; Mohan, V.; Lukashevich, V.; Odawara, M.; Paldanius, P.M.; Kothny, W. Effect of race and ethnicity on vildagliptin efficacy: A pooled analysis of phase II and III studies. Diabetes Obes. Metab. 2017, 19, 429–435.
- Klemann, C.; Wagner, L.; Stephan, M.; von Horsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21.
- Zhong, J.; Rao, X.; Deiuliis, J.; Braunstein, Z.; Narula, V.; Hazey, J.; Mikami, D.; Needleman, B.; Satoskar, A.R.; Rajagopalan, S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 2013, 62, 149–157.
- Sitagliptin: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/januvia-eparproduct-information_en.pdf (accessed on 1 August 2020).
- He, Y.L.; Wang, Y.; Bullock, J.M.; Deacon, C.F.; Holst, J.J.; Dunning, B.E.; Ligueros-Saylan, M.; Foley, J.E. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J. Clin. Pharmacol. 2007, 47, 633–641.
- Christopher, R.; Covington, P.; Davenport, M.; Fleck, P.; Mekki, Q.A.; Wann, E.R.; Karim, A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin. Ther. 2008, 30, 513–527.
- Jedlowski, P.M.; Jedlowski, M.F.; Fazel, M.T. DPP-4 Inhibitors and Increased Reporting Odds of Bullous Pemphigoid: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS) from 2006 to 2020. Am. J. Clin. Dermatol. 2021, 22, 891–900.
- Huang, J.; Jia, Y.; Sun, S.; Meng, L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: Data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol. Toxicol. 2020, 21, 68.





