Dipeptidyl peptidase 4 (DPP4) enzyme is a type II transmembrane glycoprotein, expressed ubiquitously in many tissues, including the immune cells, kidney, liver, pancreas, fat cells, and presents as a soluble form in the circulation. Dipeptidyl peptidase 4 is a serine protease, can cleave and inactivate incretin hormones, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), neuropeptides, and chemokines. In addition, DPP4 has been shown to have a direct pro-inflammatory role in lymphocytes, macrophages, and smooth muscle cell.
- DPP4i
- T2DM
- GLP1
- GIP
1. Introduction
2. DPP4 and DPP4 Inhibitions in Diabetes
2.1. Mechanisms of Effect of DPP4i
Diabetes mellitus (DM) is a worldwide health problem, which is a major cause of blindness, chronic kidney disease (CKD), stroke, lower extremity amputations, coronary heart disease and heart failure (HF) [13]. T2DM has changed from a chronic disease of the elderly in the traditional concept to a chronic disease of middle-aged and even children and adolescents [14][15][14,15]. Excess body fat along with age constitute the two most important risk factors for the premature development of T2DM [15][16][15,16]. Early onset T2DM relative to late-onset disease is associated with a more rapid deterioration of β-cell function, emphasizing the importance for early diagnosis and treatment initiation [17]. Obesity-related mechanisms that are potentially linked to the severity of the disease include adipocyte lipid spillover, ectopic fat accumulation and tissue inflammation [18]. Therapies aiming to decrease body weight are consequently a valuable strategy to delay the onset and decrease the risk of T2DM, as well as managing established disease [19]. In the past few decades, drug therapy for T2DM has developed greatly and involves several new strategies [20][21][22][20,21,22]. These new strategies include more patient-friendly ways to use the drug, such as improving weight loss. However, animal studies have demonstrated that a key barrier to the development of anti-obesity drugs is the large inability to predict human cardiovascular safety [23][24][25][23,24,25]. In tolerable doses, they rarely achieve 10% weight loss. Although the clinical success of these agents has laid the foundation for a new era of anti-obesity drugs, there is considerable debate as to how GLP1/GIP regulates metabolism and whether its receptor agonists or antagonists can be the drugs of choice for treating obesity and T2DM. At present, DPP4 inhibitors are widely used for the treatment of T2DM [26][27][28][29][26,27,28,29]. The basis for this approach lies with the finding that DPP4 has a key role in determining the clearance of the incretin hormone, GLP1 [5]. GLP1 is an intestinal peptide, which was known to have a role in glucose homeostasis via actions that include the potentiation of glucose-induced insulin secretion and the suppression of glucagon secretion [30]. Dipeptidyl peptidase 4 inhibitor (DPP4i) itself has no hypoglycemic activity. Instead, their anti-hyperglycemia effect is achieved primarily by altering levels of endogenous substrates. Once the catalytic activity of DPP4 is inhibited, the levels of these substrates change. To date, GLP1 has been considered to play a major role in the therapeutic effect of DPP4i [23]. GLP1 has been shown to be a physiological DPP4 substrate [23][25][23,25]. In vivo, endogenous levels of intact, biologically active peptides increase with DPP4 inhibition and are associated with improved glucose homeostasis [31][32][31,32]. Some studies found that GLP1 receptor antagonist inhibited GLP1 signaling pathway, and the hypoglycemic effect of DPP4i decreased [33][34][33,34], thus confirming the role of GLP1 in the mechanism of action of DPP4i. It also indicates that GLP1 is not the only regulatory factor, and even in the absence of GLP1 receptor activation, the hypoglycemic activity of DPP4i is still significant [33][34][33,34]. Another physiological substrate of DPP4 is glucose-dependent insulin polypeptide (GIP), also known as incretin, and the level of GIP increases with inhibition of DPP4 activity [35][36][35,36]. Similar to GLP-1, GIP enhances insulin secretion in pancreatic beta cells in a glucose-dependent manner but appears to act in a different way on glucagon secretion [37][38][37,38]. The response to GIP was also impaired in T2DM patients. In the past, views on the possible role of GIP in the treatment of T2DM have been largely ignored, because early studies have shown that GIP′s ability to stimulate insulin secretion is severely impaired. However, in T2DM patients, further studies to explore this problem were unable to be carried out due to the lack of appropriate GIP receptor antagonists. Recent studies have shown that GIP can improve glycemic control in patients with T2DM [39][40][39,40] and have revived studies on the development of novel antagonists [41][42][41,42]. These studies have led to a re-evaluation of the role of GIP in the anti-hyperglycemia of DPP4i. In addition, GLP1′s ability to inhibit glucagon secretion is weakened when blood glucose levels drop below normal fasting levels, while GIP enhances glucagon response to hypoglycemic levels. Thus, during insulin-induced hypoglycemia, glucagon secretion is increased due to GIP use [43]. Therefore, the increase in intact GIP levels observed after inhibition of DPP4 may help maintain the counter-regulatory response of glucagon when glucose levels are controlled at hypoglycemia [44][45][44,45]. Thus, GIP′s role in improving glucagon counter-regulation may further contribute to reducing the risk of hypoglycemia associated with DPP4i. Recent studies found the direct or indirect role of soluble DPP4 in brain, gastric, liver, kidney, adipose tissue, pancreas (with islet), cardiovascular system and muscle through GLP1/GIP signaling (Figure 1). However, whether other DPP4 substrates also contribute to the therapeutic effect of DPP4i remains to be determined. In vitro, many peptide hormones and chemokines are susceptible to DPP4 cleavage when incubated with DPP4 at high concentrations [25][45][25,45]. However, there is not much evidence that they are altered in vivo by DPP4i and there have been no adverse reactions or safety issues caused by off-target effects of DPP4i on other endogenous substrates [46][47][46,47].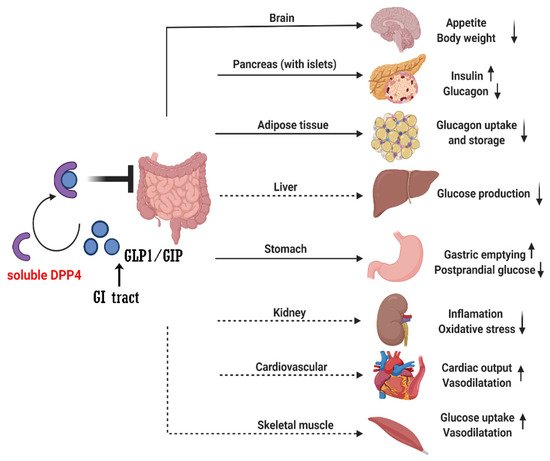
2.2. DPP4 Inhibitors
When DPP4 was identified as a therapeutic target, the search began for compounds suitable for clinical use, namely the progressive development of DPP4 inhibitors such as sitagliptin [48] and saxagliptin [49]. Currently, several structures oriented to target-specific interaction with DPP-4 are already known and officially approved by the United States Food & Drug Administration (FDA), including sitagliptin [50], saxagliptin [51], alogliptin [52], and linagliptin [53], and vildagliptin 12801240 is authorized in Europe (Table 1).|
DPP4i |
Chemistry |
Metabolism |
Half-Life |
Elimination Method |
|---|
|
DPP4i |
Trial (Year) |
Median Follow-Up, Years |
Mean/Median Age, Years |
Female (Total) |
BMI, kg/m2 * |
HbA1c, mmol/mol (%) * |
Baseline Metformin, % |
Baseline eGFR, mL/min/ [1.73 m]2 * |
Prior ASCVD, % |
Prior CHF, % |
|---|---|---|---|---|---|---|---|---|---|---|
|
Sitagliptin |
β-amino acid based |
Minimal |
||||||||
|
Sitagliptin |
|
DPP4i |
Dose (mg/Day) |
HbA1c Reduction |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
TECOS (2015) | 12.5 h |
3.0 |
Predominantly |
65 | (>80%) renal |
|||||||||
4212 | 30.2 |
55 (7.2) |
81 |
75 |
100 |
18 |
Vildagliptin |
Cyanopyrrolidine |
Hydrolysis (cytochromeindependent) to form an inactive metabolite |
~2 h | ||||
|
Sitagliptin |
100 |
0.5–1.0 | ||||||||||||
|
(14,523) | Metabolism |
(parent) and renal (metabolite) |
||||||||||||
|
Saxagliptin |
5 |
0.5–1.0 |
Saxagliptin |
Saxagliptin |
Cyanopyrrolidine |
Hydrolysis (cytochrome P450 3A4 or P450 3A5) to form an activemetabolite |
SAVOR-TIMI (2013) |
2.5 h (parent), |
2.1 |
3 h (metabolite) |
65 | |||
|
Alogliptin | Metabolism | 5590 |
31.2 |
25 (parent) and renal |
64 (8.0) |
69 |
0.6 (mean value) (metabolite) |
|||||||
73 | 78 | 13 |
Alogliptin |
Modified pyrimidinedione |
Minimal |
|||||||||
|
(16,492) | 20 h | |||||||||||||
|
Linagliptin |
5 |
0.5–0.7 | Predominantly (>70%) renal |
|||||||||||
|
Linagliptin |
Alogliptin |
Xanthine based |
EXAMINE (2013) |
Minimal |
1.5 |
~12 h (effective), >100 h (terminal) |
61 | Predominantly biliary (<6% renal) |
2.3. Benefits of DPP4i
1722 | ||||||||||
28.7 | 64 (8.0) | NA |
71 |
100 |
28 |
|||||
|
(5380) |
||||||||||
|
Linagliptin |
CARMEL (2019) |
2.2 |
66 |
2582 |
31.4 |
64 (8.0) |
54 |
55 |
57 |
27 |
NA, not available; * These are expressed as mean values.
