Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qiangbo Liu | -- | 2115 | 2022-05-19 05:44:14 | | | |
| 2 | Dean Liu | -5 word(s) | 2110 | 2022-05-20 02:48:19 | | | | |
| 3 | Dean Liu | -40 word(s) | 2070 | 2022-05-27 09:42:35 | | | | |
| 4 | Dean Liu | Meta information modification | 2070 | 2022-05-27 09:45:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Q.; , .; Su, Y.H.; Zhang, X. Genetic Mechanisms of Cold Signaling in Wheat. Encyclopedia. Available online: https://encyclopedia.pub/entry/23100 (accessed on 07 February 2026).
Liu Q, , Su YH, Zhang X. Genetic Mechanisms of Cold Signaling in Wheat. Encyclopedia. Available at: https://encyclopedia.pub/entry/23100. Accessed February 07, 2026.
Liu, Qiangbo, , Ying Hua Su, Xiansheng Zhang. "Genetic Mechanisms of Cold Signaling in Wheat" Encyclopedia, https://encyclopedia.pub/entry/23100 (accessed February 07, 2026).
Liu, Q., , ., Su, Y.H., & Zhang, X. (2022, May 19). Genetic Mechanisms of Cold Signaling in Wheat. In Encyclopedia. https://encyclopedia.pub/entry/23100
Liu, Qiangbo, et al. "Genetic Mechanisms of Cold Signaling in Wheat." Encyclopedia. Web. 19 May, 2022.
Copy Citation
Cold stress is a major environmental factor affecting the growth, development, and productivity of various crop species. With the current trajectory of global climate change, low temperatures are becoming more frequent and can significantly decrease crop yield. Wheat (Triticum aestivum L.) is the first domesticated crop and is the most popular cereal crop in the world. Because of a lack of systematic research on cold signaling pathways and gene regulatory networks, the underlying molecular mechanisms of cold signal transduction in wheat are poorly understood.
cold stress
wheat
hormonal
reactive oxygen species
1. ICE-CBF-COR Signaling Pathway in Cold Stress
ICE genes encode a class of MYC-like bHLH transcriptional factors upstream of the cold signaling pathway [1]. The C-terminal regions of ICE have highly conserved regions for specific interactions with downstream cold regulatory genes [1][2][3][4][5]. The homologs of ICE have been identified as TaICE41 and TaICE87 in wheat (Figure 1). Overexpression of TaICE41 or TaICE87 in Arabidopsis enhanced cold tolerance, suggesting the significance of ICE homologs in cold stress response [3]. HOS1 (HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1), an E3 ubiquitin ligase, reduces the stability of ICE1 protein by ubiquitination under cold stress [6]. In addition, the stability of ICE1 protein is enhanced by SUMO E3 ligase SIZ1 (SAP and Miz) through sumoylation in response to cold stress [7]. ICE1 is phosphorylated by the cold-activated protein kinase OPEN STOMATA 1 (OST1), resulting in weakened interaction between ICE1 and HOS1 to increase the stability of ICE1 under cold stress [8]. Furthermore, the stability of OsICE1 is up-regulated by OsMPK3 (MAP KINASE 3) through phosphorylation in rice in response to cold stress [9]. These results indicate that the posttranslational modification of ICE1 is crucial for its role in response to cold stress. However, whether TaICEs have similar regulatory mechanisms in wheat responses to cold stress needs further study.
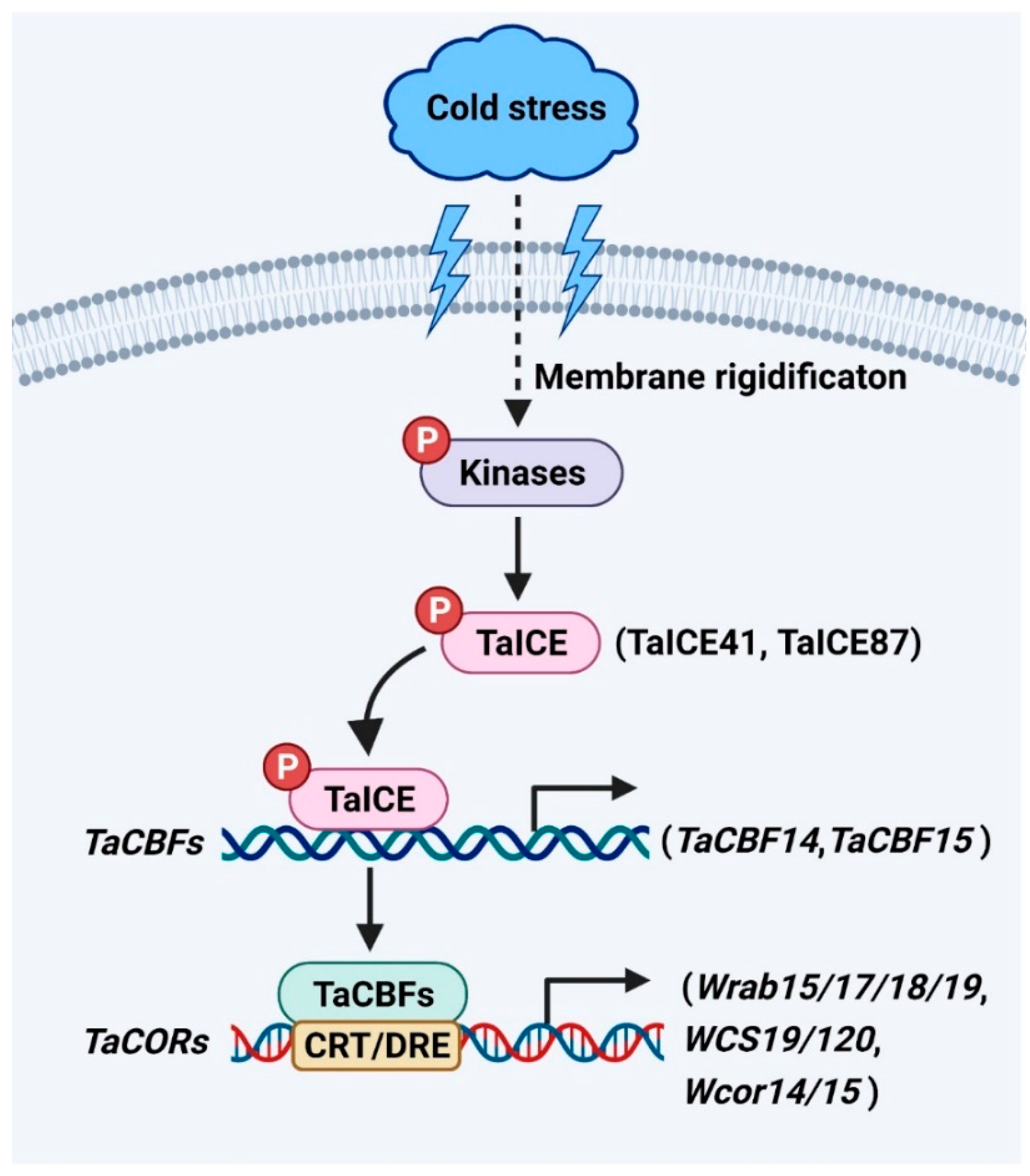
Figure 1. ICE-CBF-COR signaling pathway plays a vital role in wheat. Cold stress alters the fluidity of plasma membrane and activates protein kinases.
CBFs (CBF1, CBF2, and CBF3), which belong to the AP2/ERF multi-gene family, can be activated by ICE in the cold signaling pathway of plants [1][10]. CBFs are key components for increasing the cold tolerance of plants [11][12][13]. The overexpression of CBFs in rice, maize, barley, wheat, and other plant species significantly enhances the cold tolerance of transgenic plants [14][15][16][17][18]. However, the cbfs triple mutant in Arabidopsis show reduced cold tolerance and larger biomass than wild type [19]. These results indicate CBFs may act to balance cold tolerance and plant growth. However, whether CBFs are important regulators of growth and cold tolerance to enhance the biomass of wheat requires further study. Several CBF genes have been characterized in Triticeae species, including 37 genes from hexaploid wheat [20], 20 genes from barley [15], 13 genes from Triticum monococcum [21], 11 genes from rye [22], ten genes from durum wheat [23], ten genes from Aegilops biuncialis [2], four genes from Brachypodium distachyon [24][25], and one gene from Aegilops tauschii [20]. TaCBF14 and TaCBF15, two wheat CBF transcription factors, play significant roles in cold stress response (Figure 1) [17]. Overexpression of TaCBF14 or TaCBF15 in barley enhances the expression of HvCOR14b, a cold-regulated gene in barley, increasing cold tolerance [17]. Additionally, T. aestivum ABIOTIC STRESS-INDUCED DNA BINDING FACTOR a (TaAIDFa) is markedly activated by cold stress [26]. Overexpression of TaAIDFa in Arabidopsis increases the transcription of the cold-regulated genes like RD29A and COR15A to enhance the cold tolerance of transgenic lines [26].
CORs generally refer to the protective substances encoded by cold-regulated genes. The protective substances such as osmolytes and cryoprotective proteins accumulate to facilitate cold acclimation and freezing tolerance [27][28]. CBFs are known to bind to the C-REPEAT/DEHYDRATION RESPONSIVE ELEMENT (CRT/DRE) sequence (TACCGCAT) in the promoters of COR genes for their transcription activation in response to cold stress [29][30]. The expression of ABA-dependent COR genes (Wrab15/17/18/19) and ABA-independent COR genes (WCS19, WCS120, Wcor14, and Wcor15) are significantly increased by cold stress in wheat (Figure 1) [31]. The expression of DRE-BINDING PROTEIN 1 (TaDREB1), a wheat homolog of Arabidopsis DREB2, is elevated under cold stress [32]. The transcription of the WHEAT COLD SPECIFIC 120 (WCS120) gene is activated by TaDREB1 and increases cold tolerance in winter wheat [33]. The expression of wheat DREB2 (WDREB2), also a wheat homolog of Arabidopsis DREB2, is activated by cold [34]. The WDREB2 transcription factor directly affects the expression of wheat COR genes such as Wrab19 in response to cold stress [34].
2. Cold Stress Influences Hormonal Responses
Plant hormones (Phytohormones), which function as small molecules to regulate various cellular processes and work as chemical messengers to communicate cellular activities, are produced in very low concentrations in higher plants [35]. Phytohormones are needed for plants to deal with abiotic stresses, including salinity, drought, and low temperature, by mediating a wide range of adaptive responses [36]. These phytohormones include auxin, abscisic acid (ABA), ethylene, cytokinins (CKs), gibberellins (GAs), jasmonic acid (JA), brassinosteroids (BRs), salicylic acid (SA), and strigolactones (SLs). In recent years, the phytohormone signaling pathway has been investigated by genetic and biochemical approaches, and a growing body of evidence indicates that the elements in hormonal signaling pathways contribute to regulating plant cold tolerance [12].
Auxin, a tryptophan derivative most commonly present in the form of indole-3-acetic acid (IAA), plays an essential role in plant development and cold stress response. The YUCCA genes encode the key rate-limiting enzymes in the auxin biosynthetic pathway and are involved in the regulation of plant growth and development. The transcript levels of OsYUCCAs are strongly induced by low temperatures; however, the expression of IAA catabolism-related genes, Oryza sativa GRETCHEN HAGENs (OsGHs), is down-regulated, resulting in significantly increased IAA content in rice under cold stress (Table 1) [37]. In colder/ambient temperatures, CLAVATA (CLV) peptide signaling promotes flower development by stimulating auxin-dependent growth. In contrast, at higher temperatures, YUCCA genes are activated to maintain flower development bypass CLV signaling [38][39][40]. There are 15 genes among 63 TaYUCCAs that are induced by drought and heat stress in wheat, though it is unclear whether the expression of these genes is regulated by cold stress. Arabidopsis AUXIN RESPONSE FACTOR (ARF) genes, which regulate the expression of auxin-responsive genes by binding to the auxin response element in their promoters, are up-regulated during cold acclimation (Table 1) [41]. In wheat, 46 genes from 69 TaARFs are also up-regulated in response to cold stress (Table 1) [42].
Abscisic acid (ABA) is the most important phytohormone due to its role in plant adaptation to biotic and abiotic stresses [43]. ABA-deficient mutants in Arabidopsis show defects in freezing tolerance, with the induced expression of COR genes, suggesting that ABA is involved in cold signaling [44][45]. Additionally, ABA contents are moderately decreased after cold treatment [8]. SUCROSR NON-FERMENTING 1-RELATED PROTEIN KINASE 2s (SnRK2s) are important protein kinases in ABA signaling, and their role in abiotic and biotic stress signaling has been extensively characterized in Arabidopsis. The SnRK2 homologs in wheat appear to play a critical role in cold signaling. PKABA1, the first SnRK2 protein identified in wheat, is rapidly induced in seedlings when ABA levels increase in response to cold stress [46]. Furthermore, the expression of TaSnRK2.3, TaSnRK2.4, and TaSnRK2.8 can be induced by cold stress, suggesting that they are essential in cold signal transduction (Table 1) [47][48][49]. Overexpression of TaSnRK2.3 or TaSnRK2.8 in Arabidopsis increases cold tolerance, which is due to the increased expression of cold-responsive genes, and the enhanced accumulation of stress-associated metabolites such as proline [48]. Recent studies have identified 10 SnRK2 homologs in wheat, and the expression of these genes is induced by cold stress [50]. Although ABA and cold signaling are closely related, it is unclear what the exact role of ABA in regulating plant cold stress responses is. Further work is needed to elucidate the molecular mechanisms of ABA when regulating cold signaling pathways.
Ethylene, a gaseous plant hormone, is important in various cellular and developmental processes, as well as during abiotic and biotic stress responses [51][52][53][54][55][56]. It is reported that cold stress can alter endogenous ethylene levels in many plant species. Cold stress inhibits ethylene production in Arabidopsis [57]; however, the ethylene levels are increased in winter rye under cold stress [58]. T. aestivum ethylene-responsive factor 1 (TaERF1), the first member of the ERF gene family identified in wheat, is induced by cold stress (Table 1). Additionally, TaERF1 overexpression can activate COR genes and improve freezing tolerance in transgenic Arabidopsis [59]. Pathogen-induced ethylene response factor 1 (TaPIE1) in wheat positively regulates freezing stresses by activating cold-regulated genes downstream of the ethylene signaling pathway and by modulating related physiological traits (Table 1) [60].
Gibberellins (GAs) play vital roles in abiotic stress response and adaptation. DELLA proteins are master regulators of GA-responsive growth and development [61]. Cold stress activates the expression of GA 2-oxidase genes to reduce the content of GA, resulting in the enhanced accumulation of DELLA proteins [62]. It is reported that overexpression of CBFs reduces the bioactive GA levels to suppress plant growth and flowering. CBF1-overexpression plants exhibit dwarfism and late-flowering phenotypes due to limited accumulation of bioactive GA [62]. Additionally, the cbfs mutants display impaired cold tolerance and larger architecture than the wild type after cold acclimation [19][63]. These results indicate that both the content and signal components of GA are related to cold signaling and CBFs may be associated with GA signaling to balance low-temperature adaption and growth. DELLAs act early in the cold signaling pathway as regulators of GROWTH REGULATORY FACTORs (GRFs). Cold-induced CBF genes are decreased in GRF5-overexpression lines, indicating that GRFs can repress CBF expression under cold stress (Table 1) [64]. Overexpression of SLENDER RICE 1 (SLR1), a gene that encodes the rice DELLA protein, enhances chilling tolerance. When rice seedlings are subjected to chilling stress, the cold-induced SLR1 (Table 1) releases the repressive effect of OsGRF6 on OsGA2ox1. The increased OsGA2ox1 expression then decreases the active GA levels to enhance rice chilling tolerance [65]. Rht-B1b and Rht-D1b, the most important and common semi-dwarfing genes, encode GA-insensitive forms of DELLA proteins that likely have a reduced affinity for the GA receptor in wheat [66]. It has been reported that the Rht-B1b and Rht-D1b mutant alleles are not responsive to GA at warmer temperatures but are responsive at colder temperatures (Table 1) [67].
The phytohormone jasmonic acid (JA) and its methyl ester, methyl jasmonate (MJ), act as signaling molecules in response to environmental stimuli. Cold stress rapidly increases endogenous JA levels by up-regulating the expression of JA biosynthesis genes, such as LIPOXYGENASE 1 (LOX1), ALLENE OXIDE SYNTHASE 1 (AOS1), ALLENE OXIDE CYCLASE 1 (AOC1), JASMONATE RESISTANT 1 (JAR1) in Arabidopsis and OsLOX2, OsAOS, OsAOC, Oryza sativa 12-OXOPHYTODIENOATE REDUCTASE 1 (OsOPR1) in rice (Table 1) [37][68]. The accumulation of JA induced by cold stress is due to the repression of ICE1 by JASMONATE ZIM-DOMAIN 1/4 (JAZ1/4), repressors of jasmonate signaling, resulting in the induction of CBFs expression in Arabidopsis [68]. Wheat TaJAZ genes are up-regulated in response to low temperatures (Table 1) [69]. Additionally, endogenous JA levels increase under cold stress in wheat [70]. Exogenous MJ treatment tends to up-regulate of the transcription of COR genes, such as WCS19 and WCS120, and increase the activity of superoxide dismutase (SOD) and peroxidase (PO) to promote wheat cold tolerance [71][72]. Rice HAN1 (“han” means “chilling” in Chinese), which functions as an oxidase to reduce the accumulation of the active to inactive, decreases the expression of CBF/DREB1s in rice under cold stress [73]. Arabidopsis OPR3 is one of the major players in the JA biosynthesis pathway. Transgenic wheat plants with AtOPR3-overexpression have increased the accumulation of JA and improved cold tolerance [74].
Brassinosteroids (BRs) play a vital role in plant development and stress tolerance. COR gene expression and cold tolerance in Arabidopsis are increased by exogenous BR treatment [75]. Exogenous BR treatment promotes growth recovery of maize seedlings following chilling treatment [76] and increases cold tolerance in winter rye and winter wheat [77][78]. BRASSINOSTEROID INSENSITIVE 2 (BIN2) negatively regulates the freezing tolerance in Arabidopsis [79]. Knockout mutants of Oryza sativa GLYCOGEN SYNTHASE KINASE 3-LIKE GENE 1 (OsGSK1), an ortholog of Arabidopsis BIN2, show enhanced cold tolerance (Table 1) [80]. The expression of T. aestivum SHAGGY KINASE 5 (TaSK5), an abiotic stress-inducible GSK3/SHAGGY-like kinase in wheat, is induced at the early stages of cold acclimation (Table 1) [81]. The BRASSINOSTEROID-INSENSITIVE 1 (BRI1) encodes a transmembrane receptor kinase as a BR receptor. Its mutation results in defective BR signaling and increases cold stress tolerance in Arabidopsis (Table 1) [82]. The enhanced expression of its wheat homologous TaBRI1 in Arabidopsis leads to better cold tolerance than the wild-type plants by maintaining membrane integrity [83]. Furthermore, overexpression of TaBRI1 in Arabidopsis and the ortholog of BRI1 in rice or barley increases the silique size and seed yield [84][85].
Table 1. List of phytohormones in response to cold stress.
| Item | Gene | Function of Gene | Regulated by Cold Stress | Reference |
|---|---|---|---|---|
| Auxin | OsYUCCA2/3/6/7 | Important gene in Auxin/IPA (indole-3-pyruvic acid) biosynthesis | Up-regulated | [37] |
| OsGH3-1/2/5/6/11 | Auxin/IAA (indole-3-acetic acid) catabolism-related genes | Down-regulated | [37] | |
| ARFs | Regulate the expression of auxin-responsive genes | Up-regulated | [41] | |
| TaARFs | Regulate the expression of auxin-responsive genes | Up-regulated | [42] | |
| ABA | TaSnRK2.3/2.4/2.8 | Important serine/threonine protein kinase in ABA signaling network | Up-regulated | [47][48][49] |
| Ethylene | TaERF1 | A member of the ethylene response factor subfamily of ERF/AP2 transcription factor family | Up-regulated | [59] |
| TaPIE1 | Pathogen-induced ethylene response factor to active stress-related genes | Up-regulated | [60] | |
| Gibberellin | GRF5 | Growth regulating factor encoding transcription activator. | Up-regulated | [64] |
| SLR1 | A gene that encodes the rice DELLA protein to active OsGA2ox1 expression | Up-regulated | [65] | |
| Rht-B1b, Rht-D1b |
The most important and widely used semi-dwarfing genes | Up-regulated | [67] | |
| Jasmonic acid | LOX1, AOS1, AOC1, JAR1 | JA biosynthesis genes in Arabidopsis | Up-regulated | [37] |
| OsLOX2, OsAOS, OsAOC, OsOPR1 | JA biosynthesis genes in rice | Up-regulated | [68] | |
| TaJAZs | The repressors of jasmonate signaling | Up-regulated | [69] | |
| Brassinosteroids | OsGSK1 | BR negative regulator | Up-regulated | [80] |
| TaSK5 | An abiotic stress-inducible GSK3 in wheat | Up-regulated | [81] | |
| TaBRI1 | BR receptor | Up-regulated | [82] |
References
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.H.; Agarwal, M.; Zhu, J.K. ICE1, a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054.
- Jin, Y.N.; Zhai, S.S.; Wang, W.J.; Ding, X.H.; Guo, Z.F.; Bai, L.P.; Wang, S. Identification of genes from the ICE-CBF-COR pathway under cold stress in Aegilops-Triticum composite group and the evolution analysis with those from Triticeae. Physiol. Mol. Biol. Plants 2018, 24, 211–229.
- Badawi, M.; Reddy, Y.V.; Agharbaoui, Z.; Tominaga, Y.; Danyluk, J.; Sarhan, F.; Houde, M. Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol. 2008, 49, 1237–1249.
- Lee, B.H.; Henderson, D.A.; Zhu, J.K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175.
- Lu, X.; Yang, L.; Yu, M.Y.; Lai, J.B.; Chao Wang, C.; McNeil, D.; Zhou, M.X.; Yang, C.W. A novel Zea mays ssp. mexicana L. MYC-type ICE-like transcription factor gene ZmmICE1, enhances freezing tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 113, 78–88.
- Dong, C.H.; Agarwal, M.; Zhang, Y.Y.; Xie, Q.; Zhu, J.K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 2006, 103, 8281–8286.
- Miura, K.; Jin, J.B.; Lee, J.Y.; Yoo, C.Y.; Stirm, V.; Miura, T.; Ashworth, E.N.; Bressan, R.A.; Yun, D.J.; Hasegawa, P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 2007, 19, 1403–1414.
- Ding, Y.L.; Li, H.; Zhang, X.Y.; Xie, Q.; Gong, Z.Z.; Yang, S.H. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289.
- Zhang, Z.Y.; Li, J.H.; Li, F.; Liu, H.H.; Yang, W.S.; Chong, K.; Xu, Y.Y. OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev. Cell 2017, 43, 731–743.
- Shi, Y.T.; Ding, Y.L.; Yang, S.H. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637.
- Park, S.C.; Lee, C.M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.S.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207.
- Shi, Y.T.; Ding, Y.L.; Yang, S.H. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015, 56, 7–15.
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Wang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559.
- Qin, F.; Sakuma, Y.; Li, J.; Liu, Q.; Li, Y.Q.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004, 45, 1042–1052.
- Skinner, J.S.; von Zitzewitz, J.; Szucs, P.; Marquez-Cedillo, L.; Filichkin, T.; Amundsen, K.; Stockinger, E.J.; Thomashow, M.F.; Chen, T.H.; Hayes, P.M. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Mol. Biol. 2005, 59, 533–551.
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153.
- Soltész, A.; Smedley, M.; Vashegyi, I.; Galiba, G.; Harwood, W.; Vágújfalvi, A. Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J. Exp. Bot. 2013, 64, 1849–1862.
- Masoomi-Aladizgeh, F.; Aalami, A.; Esfahani, M.; Aghaei, M.J.; Mozaffari, K. Identification of CBF14 and NAC2 genes in Aegilops tauschii associated with resistance to freezing stress. Appl. Biochem. Biotechnol. 2015, 176, 1059–1070.
- Jia, Y.X.; Ding, Y.L.; Shi, Y.T.; Zhang, X.Y.; Gong, Z.Z.; Yang, S.H. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353.
- Badawi, M.; Danyluk, J.; Boucho, B.; Houde, M.; Sarhan, F. The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexity of cereal CBFs. Mol. Genet. Genom. 2007, 277, 533–554.
- Miller, A.K.; Galiba, G.; Dubcovsky, J. A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am2 in Triticum monococcum. Mol. Genet. Genom. 2006, 275, 193–203.
- Siddiqua, M.; Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011, 34, 1345–1359.
- Leonardis, A.M.D.; Marone, D.; Mazzucotelli, E.; Neffar, F.; Rizza, F.; Fonzo, N.D.; Cattivelli, L.; Mastrangelo, A.M. Durum wheat genes up-regulated in the early phases of cold stress are modulated by drought in a developmental and genotype dependent manner. Plant Sci. 2007, 172, 1005–1016.
- Li, C.; Rudi, H.; Stockinger, E.J.; Cheng, H.M.; Cao, M.J.; Fox, S.E.; Mockler, T.C.; Westereng, B.; Fjellheim, S.; Rognli, O.A.; et al. Comparative analyses reveal potential uses of Brachypodium distachyon as a model for cold stress responses in temperate grasses. BMC Plant Biol. 2012, 12, 65.
- Ryu, J.Y.; Hong, S.Y.; Jo, S.H.; Woo, J.C.; Lee, S.; Park, C.M. Molecular and functional characterization of cold-responsive C-repeat binding factors from Brachypodium distachyon. BMC Plant Biol. 2014, 14, 15.
- Xu, Z.S.; Ni, Z.Y.; Liu, L.; Nie, L.N.; Li, L.C.; Chen, M.; Ma, Y.Z. Characterization of the TaAIDFa gene encoding a CRT/DRE-binding factor responsive to drought, high-salt, and cold stress in wheat. Mol. Genet. Genom. 2008, 280, 497–508.
- Ding, Y.L.; Shi, Y.T.; Yang, S.H. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564.
- Guo, J.; Ren, Y.K.; Tang, Z.H.; Shi, W.P.; Zhou, M.X. Characterization and expression profiling of the ICE-CBF-COR genes in wheat. PeerJ 2019, 7, e8190.
- Shi, Y.H.; Huang, J.Y.; Sun, T.S.; Wang, X.F.; Zhu, C.Q.; Ai, Y.X.; Gu, H.Y. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133.
- Song, Y.; Zhang, X.Y.; Li, M.Z.; Yang, H.; Fu, D.Y.; Lv, J.; Ding, Y.L.; Gong, Z.Z.; Shi, Y.T.; Yang, S.H. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021, 63, 1874–1887.
- Sun, X.C.; Hu, C.X.; Tan, Q.L.; Liu, J.S.; Liu, H.E. Effects of molybdenum on expression of cold-responsive genes in abscisic acid (ABA)-dependent and ABA-independent pathways in winter wheat under low-temperature stress. Ann. Bot. 2009, 104, 345–356.
- Shen, Y.G.; Zhang, W.K.; He, S.J.; Zhang, J.S.; Liu, Q.; Chen, S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930.
- Ouellet, F.; Vazquez-Tello, A.; Sarhan, F. The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Lett. 1998, 423, 324–328.
- Egawa, C.; Kobayashi, F.; Ishibashi, M.; Nakamura, T.; Nakamura, C.; Takumi, S. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 2006, 81, 77–91.
- Voß, U.; Bishopp, A.; Farcot, E.; Bennett, M.J. Modelling hormonal response and development. Trends Plant Sci. 2014, 19, 311–319.
- Weijers, D.; Wagner, D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574.
- Du, H.; Liu, H.B.; Xiong, L.Z. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397.
- Sun, J.Q.; Qi, L.L.; Li, Y.N.; Chu, J.F.; Li, C.Y. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet. 2012, 8, e1002594.
- Franklin, K.A.; Lee, S.H.; Patel, D.; Kumar, S.V.; Spartz, A.K.; Gu, C.; Ye, S.Q.; Yu, P.; Breen, G.; Cohen, J.D.; et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 2011, 108, 20231–20235.
- Jones, D.S.; John, A.; VanDerMolen, K.R.; Nimchuk, Z.L. CLAVATA signaling ensures reproductive development in plants across thermal environments. Curr. Biol. 2021, 31, 220–227.
- Hannah, M.A.; Heyer, A.G.; Hincha, D.K. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 2005, 1, e26.
- Xu, L.; Wang, D.Z.; Liu, S.; Fang, Z.F.; Su, S.C.; Guo, C.M.; Zhao, C.P.; Tang, Y.M. Comprehensive atlas of wheat (Triticum aestivum L.) AUXIN RESPONSE FACTOR expression during male reproductive development and abiotic stress. Front. Plant Sci. 2020, 11, 586144.
- Wania, S.H.; Kumarb, V.; Shriramc, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176.
- Mantyla, E.; Lang, V.; Palva, E.T. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995, 107, 141–148.
- Xiong, L.M.; Ishitani, M.; Lee, H.; Zhu, J.K. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 2001, 13, 2063–2083.
- Holappa, L.D.; Walker-Simmons, M.K. The wheat abscisic acid-responsive protein kinase mRNA, PKABA1, is up-regulated by dehydration, cold temperature, and osmotic stress. Plant Physiol. 1995, 108, 1203–1210.
- Mao, X.G.; Zhang, H.Y.; Tian, S.J.; Chang, X.P.; Jing, R.L. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696.
- Zhang, H.Y.; Mao, X.G.; Wang, C.S.; Jing, R.L. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE 2010, 5, e16041.
- Tian, S.J.; Mao, X.G.; Zhang, H.Y.; Chen, S.S.; Zhai, C.C.; Yang, S.M.; Jing, R.L. Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat. J. Exp. Bot. 2013, 64, 2063–2080.
- Zhang, H.Y.; Li, W.Y.; Mao, X.G.; Jing, R.L.; Jia, H.F. Differential activation of the wheat SnRK2 family by abiotic stresses. Front. Plant Sci. 2016, 7, 420.
- Kim, C.Y.; Liu, Y.D.; Thorne, E.T.; Yang, H.P.; Fukushige, H.; Gassmann, W.; Hildebrand, D.; Sharp, R.E.; Zhang, S.Q. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 2003, 15, 2707–2718.
- Zhao, X.C.; Schaller, G.E. Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS Lett. 2004, 562, 189–192.
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.R.; Harberd, N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006, 311, 91–94.
- Cao, W.H.; Liu, J.; He, X.J.; Mu, R.L.; Zhou, H.L.; Chen, S.Y.; Zhang, J.S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719.
- Wang, Y.N.; Liu, C.; Li, K.X.; Sun, F.F.; Hu, H.Z.; Li, X.; Zhao, Y.K.; Han, C.Y.; Zhang, W.S.; Duan, Y.F.; et al. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol. Biol. 2007, 64, 633–644.
- Chen, H.M.; Xue, L.; Chintamanani, S.; Germain, H.; Lin, H.Q.; Cui, H.T.; Cai, R.; Zuo, J.R.; Tang, X.Y.; Li, X.; et al. ETHYLENE INSENSITIVE 3 and ETHYLENE INSENSITIVE3-LIKE 1 repress SALICYLIC ACID INDUCTION DEFICIENT 2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 2009, 21, 2527–2540.
- Shi, Y.T.; Tian, S.W.; Hou, L.Y.; Huang, X.Z.; Zhang, X.Y.; Guo, H.W.; Yang, S.H. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595.
- Yu, X.M.; Griffith, M.; Wiseman, S.B. Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol. 2001, 126, 1232–1240.
- Xu, Z.S.; Xia, L.Q.; Chen, M.; Cheng, X.G.; Zhang, R.Y.; Li, L.C.; Zhao, Y.X.; Lu, Y.; Ni, Z.Y.; Liu, L.; et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol. Biol. 2007, 65, 719–732.
- Zhu, X.L.; Qi, L.; Liu, X.; Cai, S.B.; Xu, H.J.; Huang, R.F.; Li, J.R.; Wei, X.N.; Zhang, Z.Y. The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol. 2014, 164, 1499–1514.
- Zentella, R.; Hu, J.H.; Hsieh, W.P.; Matsumoto, P.A.; Dawdy, A.; Barnhill, B.; Oldenhof, H.; Hartweck, L.M.; Maitra, S.; Thomas, S.G.; et al. O-GlcNAcylation of master growth repressor DELLA by SECRET AGENT modulates multiple signaling pathways in Arabidopsis. Genes Dev. 2016, 30, 164–176.
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129.
- Ding, Y.L.; Yang, S.H. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958.
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. GROWTH-REGULATING FACTORS interact with DELLAs and regulate growth in cold stress. Plant Cell 2020, 32, 1018–1034.
- Li, Z.T.; Wang, B.; Zhang, Z.Y.; Luo, W.; Tang, Y.Y.; Niu, Y.D.; Chong, K.; Xu, Y.Y. OsGRF6 interacts with SLR1 to regulate OsGA2ox1 expression for coordinating chilling tolerance and growth in rice. J. Plant Physiol. 2021, 260, 153406.
- Tang, T.; Botwright Acuña, T.; Spielmeyer, W.; Richards, R.A. Effect of gibberellin-sensitive Rht18 and gibberellin-insensitive Rht-D1b dwarfing genes on vegetative and reproductive growth in bread wheat. J. Exp. Bot. 2021, 72, 445–458.
- Pereira, M.J.; Pfahler, P.T.; Barnett, R.D.; Blount, A.R.; Wofford, D.S.; Littell, R.C. Coleoptile length of dwarf wheat isolines: Gibberellic acid, temperature, and cultivar interactions. Crop Sci. 2002, 42, 1483–1487.
- Hu, Y.R.; Jiang, L.Q.; Wang, F.; Yu, D.Q. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor 1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924.
- Wang, Y.K.; Qiao, L.Y.; Bai, J.F.; Wang, P.; Duan, W.J.; Yuan, S.H.; Yuan, G.L.; Zhang, F.T.; Zhang, L.P.; Zhao, C.P. Genome-wide characterization of JASMONATE-ZIM DOMAIN transcription repressors in wheat (Triticum aestivum L.). BMC Genom. 2017, 18, 152.
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576.
- Talanova, V.; Titov, A.; Repkina, N.; Ignatenko, A. Effect of methyl jasmonate on the expression of Wcs genes and the activity of antioxidant enzymes at wheat cold adaptation. Dokl. Biochem. Biophys. 2018, 482, 238–241.
- Repkina, N.; Ignatenko, A.; Holoptseva, E.; MiszalskI, Z.; Kaszycki, P.; Talanova, V. Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants 2021, 10, 1421.
- Mao, D.H.; Xin, Y.Y.; Tan, Y.J.; Hu, X.J.; Bai, J.J.; Liu, Z.Y.; Yu, Y.L.; Li, L.Y.; Peng, C.; Fan, T.; et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc. Natl. Acad. Sci. USA 2019, 116, 3494–3501.
- Pigolev, A.V.; Miroshnichenko, D.N.; Pushin, A.S.; Terentyev, V.V.; Boutanayev, A.M.; Dolgov, S.V.; Savchenko, T.V. Overexpression of Arabidopsis OPR3 in hexaploid wheat (Triticum aestivum L.) alters plant development and freezing tolerance. Int. J. Mol. Sci. 2018, 19, 3989.
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364.
- Krishna, P. Brassinosteroid-mediated stress responses. J. Plant Growth Regul. 2003, 22, 289–297.
- Janeczko, A.; Pociecha, E.; Dziurka, M.; Jurczyk, B.; Libik-Konieczny, M.; Oklestkova, J.; Novák, O.; Pilarska, M.; Filek, M.; Rudolphi-Skórska, E.; et al. Changes in content of steroid regulators during cold hardening of winter wheat-Steroid physiological/biochemical activity and impact on frost tolerance. Plant Physiol. Biochem. 2019, 139, 215–228.
- Sadura, I.; Janeczko, A. Brassinosteroids and the tolerance of cereals to low and high temperature stress: Photosynthesis and the physicochemical properties of cell membranes. Int. J. Mol. Sci. 2021, 23, 342.
- Ye, K.Y.; Li, H.; Ding, Y.L.; Shi, Y.T.; Song, C.P.; Gong, Z.Z.; Yang, S.H. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696.
- Koh, S.; Lee, S.C.; Kim, M.K.; Koh, J.H.; Lee, S.; An, G.; Choe, S.; Kim, S.R. T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 2007, 65, 453–466.
- Christov, N.K.; Christova, P.K.; Kato, H.; Liu, Y.L.; Sasaki, K.; Imai, R. TaSK5, an abiotic stress-inducible GSK3/shaggy-like kinase from wheat, confers salt and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 84, 251–260.
- Kim, S.Y.; Kim, B.H.; Nam, K.H. Reduced expression of the genes encoding chloroplast-localized proteins in a cold-resistant bri1 (brassinosteroid-insensitive 1) mutant. Plant Signal. Behav. 2010, 5, 458–463.
- Singh, A.; Breja, P.; Khurana, J.P.; Khurana, P. Wheat Brassinosteroid-Insensitive 1 (TaBRI1) interacts with members of TaSERK gene family and cause early flowering and seed yield enhancement in Arabidopsis. PLoS ONE 2016, 11, e0153273.
- Chono, M.; Honda, I.; Zeniya, H.; Yoneyama, K.; Saisho, D.; Takeda, K.; Takatsuto, S.; Hoshino, T.; Watanabe, Y. A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol. 2003, 133, 1209–1219.
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006, 141, 924–931.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
27 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




