Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malka Cohen-Armon | -- | 2049 | 2022-05-18 12:07:35 | | | |
| 2 | Conner Chen | -22 word(s) | 2027 | 2022-05-19 03:08:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cohen-Armon, M. A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression. Encyclopedia. Available online: https://encyclopedia.pub/entry/23066 (accessed on 07 February 2026).
Cohen-Armon M. A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression. Encyclopedia. Available at: https://encyclopedia.pub/entry/23066. Accessed February 07, 2026.
Cohen-Armon, Malka. "A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression" Encyclopedia, https://encyclopedia.pub/entry/23066 (accessed February 07, 2026).
Cohen-Armon, M. (2022, May 18). A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression. In Encyclopedia. https://encyclopedia.pub/entry/23066
Cohen-Armon, Malka. "A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression." Encyclopedia. Web. 18 May, 2022.
Copy Citation
PolyADP-ribosylation is an evolutionary conserved, reversible post-translational modification of proteins. Numerous nuclear proteins act as substrates of the abundant nuclear polyADP-ribose polymerase 1 (PARP1). In this modification, negatively charged ADP-ribose chains constructed on chromatin-bound proteins, cause their repulsion from the negatively charged DNA. In accordance, polyADP-ribosylation is a post-translational modification of proteins that causes relaxation of the highly condensed structure of the chromatin. Histone H1, which is bound to the linker DNA, located between the nucleosomes, is a prominent substrate of PARP1.
PARP1 activation
H1 polyADP-ribosylation
signal transduction pathways
1. Introduction
PolyADP-ribosylation is an evolutionary conserved, reversible post-translational modification of proteins. Numerous nuclear proteins act as substrates of the abundant nuclear polyADP-ribose polymerase 1 (PARP1) [1][2][3][4][5]. In this modification, negatively charged ADP-ribose chains constructed on chromatin-bound proteins, cause their repulsion from the negatively charged DNA [1][2][3][4][5]. In accordance, polyADP-ribosylation is a post-translational modification of proteins that causes relaxation of the highly condensed structure of the chromatin [6][7][8][9]. Histone H1, which is bound to the linker DNA, located between the nucleosomes, is a prominent substrate of PARP1. Recent findings identified eviction of polyADP-ribosylated H1 from the linker DNA in response to a variety of stimulations, resulting in DNA relaxation [8][9][10][11][12][13].
Chromatin relaxation due to polyADP-ribosylation is implicated in DNA repair. The abundant polyADP-ribose polymerase 1 (PARP1) is a major player in the initiation of DNA repair [1][2][3][4][5]. PARP1 is rapidly polyADP-ribosylated by binding to DNA breaks. PARP1 polyADP-ribosylation induces chromatin relaxation near the breaks, and the constructed ADP-ribose polymers are implicated in the recruitment of DNA-repairing enzymes to DNA breaks [1][2][3][4][5].
However, recent reports disclosed that PARP1 is also activated in the absence of DNA damage. In one mechanism, PARP1 was activated downstream to intracellular signal transduction mechanisms in a variety of cell types [10][14][15][16]. The binding of growth factors, transmitters, or hormones to their receptors in the cell membrane, as well as membrane depolarization in excitable cells, activated PARP1 via networks of intracellular signal transduction pathways, causing chromatin relaxation by polyADP-ribosylation of PARP1 and its substrate linker histone H1 [10][14][15][16]. A long-lasting polyADP-ribosylation of PARP1 was measured downstream to the MAP kinase cascade activation, and PARP1 polyADP-ribosylation mediated the phosphorylation of transcription factors and the acetylation of core histones promoting Erk-induced gene expression [10][14][15][16][17][18].
2. PARP1 Activation by Ca2+ Released from Intracellular IP3-Gated Stores
In a variety of cell types treated with hormones, growth factors, neurotransmitters, and membrane depolarization in excitable cells, G-protein-coupled receptors and receptor tyrosine kinases are activated [19][20]. Their activation initiates the activation of a variety of second messengers in networks of signal transduction pathways involving protein kinases and phosphatases [19][20]. In one of these signaling mechanisms, the multi-targeted phospholipase-C cleaves phosphatidyl inositols that are embedded in the cell membrane, including phosphatidylinositol 4,5-bisphosphate (PIP2), which is cleaved into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) [19][20][21]. DAG remains embedded in the membrane and acts as an anchoring molecule, and IP3 is released into the cytoplasm [19][20][21].
The binding of IP3 to specific receptors in Ca2+ gated stores in the endoplasmic reticulum induces intracellular Ca2+ release [21][22][23][24]. IP3-induced Ca2+ release from its stores in the endoplasmic reticulum, which includes the perinuclear membrane, activates a variety of Ca2+-dependent enzymes, including kinases and phosphatases participating in a variety of signal transduction pathways [22][23][24][25]. PARP1 was activated in response to Ca2+ release from the perinuclear stores into the nucleoplasm [25][26]. Ca-induced PARP1 polyADP-ribosylation was measured in isolated nuclei of a variety of cell types [25][26]. The effect of Ca2+ on PARP1 polyADP-ribosylation resembled the well-documented co-factor activity of Mg2+ ions in in-vitro polyADP-ribosylation of PARP1 [26]. Divalent cations frequently act as co-factors in enzymatic reactions [27].
The IP3-induced release of Ca2+ into the nucleoplasm enhanced the polyADP-ribosylation of PARP1 in nuclei of rat cerebral neurons, as well as in nuclei of neuronal cells of the marine slug Aplysia [25] (Figure 1).
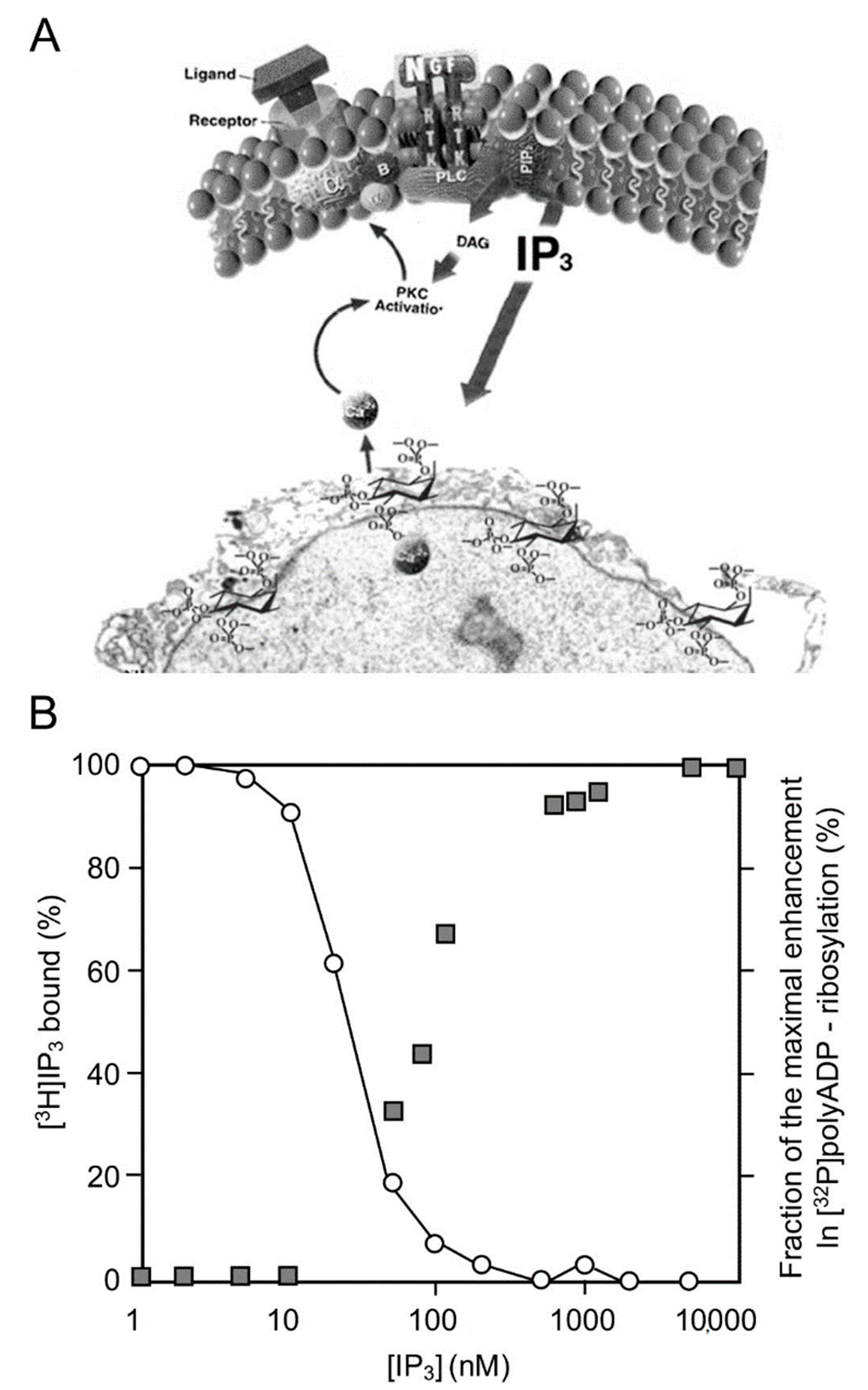
Figure 1. PARP1 activation by Ca2+ release from IP3-gated stores: (A) a schematic presentation of IP3 production by the cleavage of a phosphatidylinositol (PIP2) in the cell membrane, to DAG (diacylglycerol) and IP3 (Inositol triphosphate). IP3 induces Ca2+ release from IP3-gated Ca2+ stores in the perinuclear membrane of isolated nucleus (an electro-micrograph); (B) IP3-induced Ca2+ release activates PARP1. Right ordinate: a dose–response curve of IP3-induced [32P]polyADP-ribosylation of PARP1 in isolated nuclei of rat cerebral neurons, as measured by autography of [32P]labeled polyADP-ribosylated PARP1 immunoblots. Left ordinate: dose-dependent displacement of [3H]IP3 from specific IP3 receptors in the perinuclear membrane of isolated nuclei by unlabeled IP3 [26].
PARP1 was dose-dependently and instantaneously activated by IP3-induced Ca2+ -release into the nucleoplasm [26]. In addition, PARP1 was activated downstream to Ca-dependent activation of kinases, including activation of PKC and the calmodulin-dependent CAM kinases that are implicated in gene expression [28][29][30].
PKC is activated by numerous signal transduction mechanisms in a variety of cell types, including malignant cells [30][31][32]. Downstream, PKC phosphorylates RAF kinases that phosphorylate MEK kinases. MEK phosphorylation initiates the activation of the MAP kinase phosphorylation cascade, which is implicated in numerous signal transduction pathways in the cell [30][31][32].
3. A Long-Lasting PARP1 Activation by Phosphorylated Erk
Signals inducing phosphorylation of MEK dimers, which are bound to dimers of Erk (extracellular signal-regulated kinase) in the cytoplasm, release the phosphorylated Erk [32][33][34][35]. The released phosphorylated Erk, which lacks the nuclear localization signal (NLS), is translocated into the nucleus [33][34][35][36]. Phosphorylated Erk has a long-lasting activity in the nucleus, despite lacking the NLS signal [35][36]. A variety of transcription factors promoting the expression of numerous genes are activated by Erk-induced phosphorylation [35][37], including those promoting immediate-early genes (IEG) expression [37][38].
Phosphorylated Erk1/2 are apparently translocated into the nucleus as homodimers [33][34] by specific transporters [33][34][35][36]. In view of the similarities between the protein binding sites of Erk1 and Erk2, as well as the major role of Erk2 activity in mammals [39], the effect of phosphorylated Erk2 on PARP1 activity has been explored. Co-immunoprecipitation of phosphorylated Erk2 with PARP1 or polyADP-ribosylated PARP1 was measured in a variety of cell types [10][15][40]. Furthermore, consensus docking sites of phosphorylated Erk were identified in PARP1 [10]. They include four sites that partially match the known docking motifs of phosphorylated Erk, found in its substrates 633KYPKK637, 683KK684, 747KKPPLL752, and 1007FNF. They are located in the WGR domain, helical domain (HD), and catalytic (CAT) domain of PARP1 (aa556–1014) [10][41][42][43][44]. In addition, a negatively charged protein-binding domain in Erk (CRS/CD region) was involved in its binding to the docking sites in PARP1 [10][15] (Figure 2). Phosphorylated Erk was co-immunoprecipitated with PARP1 in nuclear extracts of a variety of stimulated cell types, and MEK inhibitors interfered with their binding [10][15][16]. Furthermore, in a cell-free system, recombinant PARP1 was activated and highly polyADP-ribosylated upon binding of recombinant phosphorylated Erk2 [10][15]. In accordance, PARP1 activation measured in a variety of stimulated cell types was dependent on the phosphorylation of MEK [10][14][15][16][40] (Figure 2C). In addition, a long-lasting activity of phosphorylated Erk in the nuclei of these cells was dependent on PARP1 [10][15][16][17][18]. A variety of transcription factors, promoters, and modulators in the chromatin, which are prominent substrates of phosphorylated Erk, were hardly phosphorylated in PARP1 KO cells [10][18], indicating the PARP1-dependent activity of phosphorylated Erk in the chromatin. PolyADP-ribosylated PARP1 apparently acts as a platform for phosphorylated Erk, enabling phosphorylation of Erk substrates while causing local chromatin relaxation.
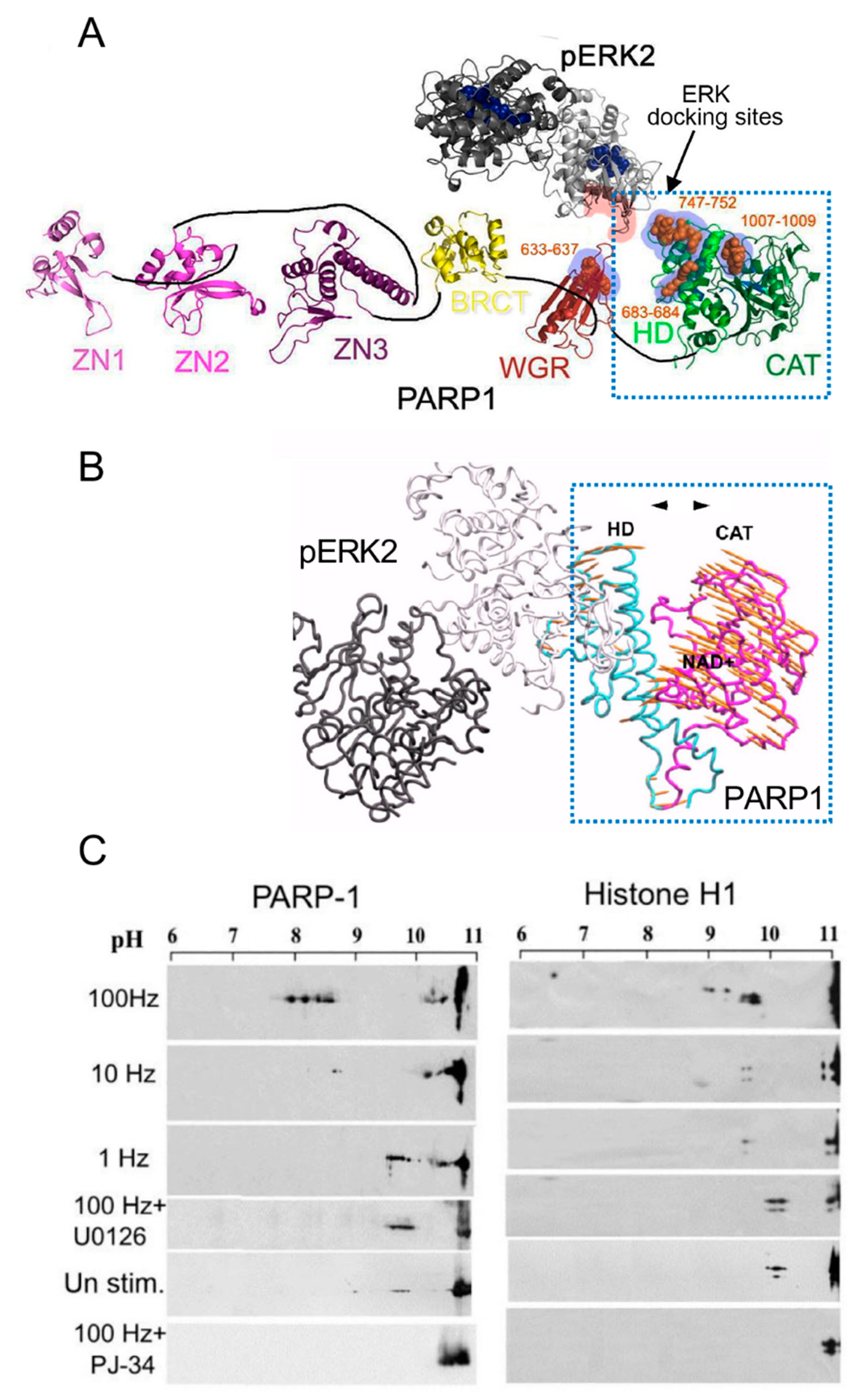
Figure 2. PARP1 is activated by binding to phosphorylated Erk: (A) a Ribbon structural model of the open conformation of PARP1 with optional consensus docking sites for phosphorylated Erk. Erk2 monomers in a homodimer (formed after Erk2 phosphorylation, PDB 2ERK) are indicated by dark and light gray ribbons. The potential Erk-binding motifs in the HD, WGR, and the CAT domain of PARP1 are indicated by orange spheres. The CRS/CD protein-binding region in Erk2 and the optional Erk-binding motifs in PARP1 are highlighted by red and blue shadows, respectively; (B) a model based on the calculated intra-molecular movements in PARP1 bound to homodimer of phosphorylated Erk2, predicting that the helical (HD) and the catalytic (CAT) domains of PARP1 move to opposite directions (yellow arrows), thereby exposing the NAD binding site in the CAT domain of PARP1; (C) polyADP-ribosylation of PARP1 and histone H1 in electrically stimulated rat cerebral neurons in primary culture. PolyADP-ribosylation of the proteins in the stimulated cerebral neurons was measured by the shift in their isoelectric points on 2D gels, which is prevented in neurons treated with either PARP or MEK inhibitors (PJ34 or U0126, respectively) [10].
Notably, in a cell-free system, recombinant PARP1 was activated and polyADP-ribosylated by a recombinant phosphorylated Erk that lacks its kinase activity [15], indicating that the activation of Erk-bound PARP1 does not involve PARP1 phosphorylation [10][15]. Recombinant PARP1 bound to phosphorylated Erk2 was apparently activated due to intra-molecular modifications in PARP1, exposing the NAD+ binding site in its catalytic domain [10][15][44] (Figure 2A,B). Accordingly, the calculated affinity for NAD+ of recombinant PARP1 bound to recombinant phosphorylated Erk2 was about 70 times higher than the affinity of DNA-bound recombinant PARP1 [15]. In addition, both PARP1 and polyADP-ribosylated PARP1 equally bound phosphorylated Erk2 [10][15], outlining a mechanism that keeps PARP1 activated as long as PARP1 is bound to phosphorylated Erk [10][15][18].
The binding of PARP1 to phosphorylated Erk did not interfere with PARP1 binding to its substrate histone H1 [10][14][15][16]. Thus, the accessibility of PARP1-bound phosphorylated Erk to its substrates in the chromatin could be attributed to a local chromatin relaxation due to histone H1 polyADP-ribosylation by activated PARP1-bound to phosphorylated Erk [10][14][15]. This assumption was further supported by documented eviction of polyADP-ribosylated histone H1 from its sites in the linker DNA in response to depolarization of cerebral neurons or in response to receptors activation in the cell membrane [9][12][13][16]. Accordingly, Erk-dependent immediate-early gene expression was extensively downregulated in neurons of PARP1-KO mice, or after PARP1 silencing [10][15][18][40]. Additionally, a relatively long-lasting activity of phosphorylated Erk2 was documented in the nucleus of normal cells, compared with that period in cells prepared from PARP1 KO mice [18]. The long-lasting activity of phosphorylated Erk in the nucleus is one of the unresolved phenomena in cell biology [35][36][38]. The long-lasting binding of PARP1 to phosphorylated Erk presents a possible mechanism explaining this unresolved phenomenon [10][14][15][18][36].
The expression of immediate-early genes was dependent on activities of both phosphorylated Erk2 and PARP1 [10][15][16][18] and was accompanied by the eviction of polyADP-ribosylated histone H1 rendering transcription factors accessible to phosphorylation by PARP1-bound phosphorylated Erk2 [10][12][14][15]. Thus, when PARP1 is activated by a variety of signal transduction mechanisms, activating the MAP kinase phosphorylation cascade in response to extracellular signals, the synergism between PARP1 and phosphorylated Erk mediates Erk-induced gene expression (Figure 2C, Figure 3 and Figure 4A).
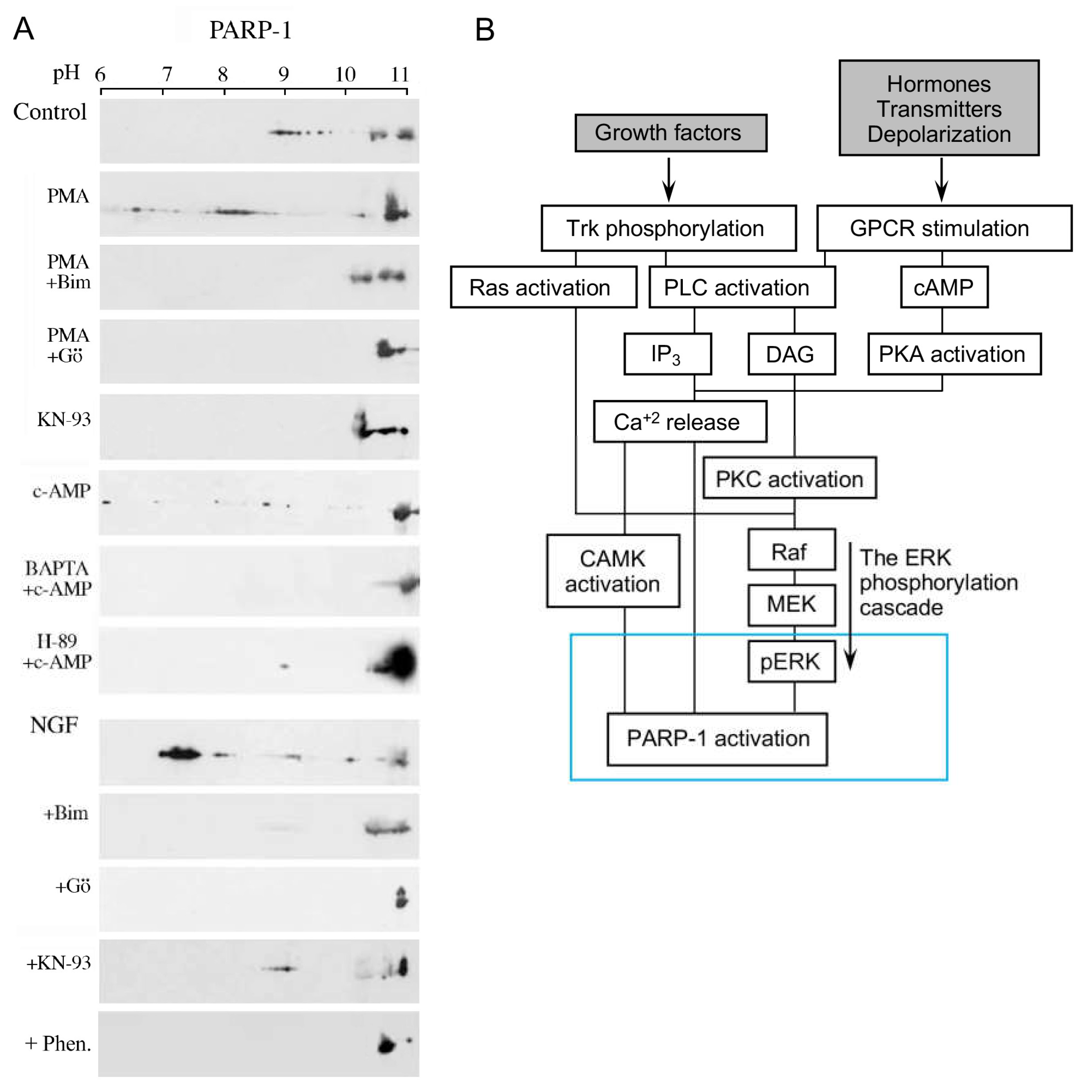
Figure 3. Activation and polyADP-ribosylation of PARP1 via signal-transduction Mechanisms: (A) PARP1 activation by signal transduction mechanisms activating G-protein-coupled receptors and receptor tyrosine kinase (TrkA), PKC, CAMKII, and PKA. The polyADP-ribosylation of PARP1 was measured by the shift in its isoelectric point on 2D gels, which is prevented by PARP inhibition (6(5H)-phenanthridinone (Phen)). The polyADP-ribosylation of PARP1 was induced in neurons treated with the indicated nerve growth factor (NGF), PKC activator (phorbol ester PMA), and PKA activator (cAMP). PARP1-induced polyADP-ribosylation was inhibited by treatments with the inhibitors of the indicated kinase PKC inhibitors, Go6796 and Bim-1, CAMK inhibitor KN-93, calcium chelator BAPTA, and PKA inhibitor H-89; (B) a flowchart presentation of signal transduction mechanisms mediating PARP1 activation in response to extracellular stimulations [16][17].
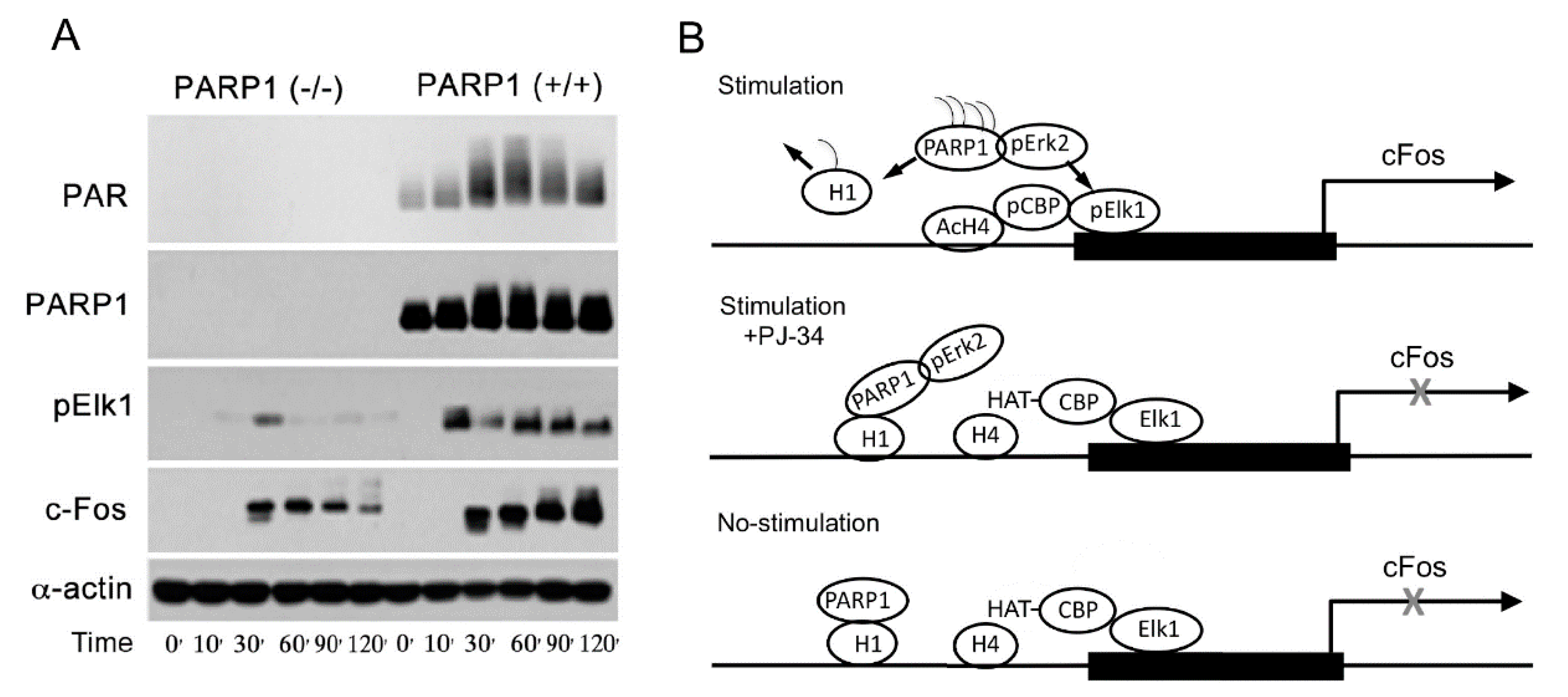
Figure 4. Elk phosphorylation and cfos expression depend on the expression of PARP1: (A) the presented immunoblots indicate time-dependent changes in the levels of PARP1, PAR, phosphorylated Elk1, and the protein c-Fos, following treatment of mouse embryonic fibroblasts (MEF) and PARP1-KO MEF with the PKC activator PMA; (B) a schematic description of PARP1-dependent expression of cfos. Binding of phosphorylated Erk to PARP1 activates PARP1 and polyADP-ribosylates PARP1 and linker histone H1. PolyADP-ribosylation of H1, resulting in its removal from the linker DNA, causes chromatin relaxation that facilitates the phosphorylation of transcription factor Elk1 by phosphorylated Erk bound to polyADP-ribosylated PARP1. Elk1 phosphorylation induces CBP/p300 phosphorylation that evokes their HAT activity. The resulting core histone H4 acetylation promotes c-fos expression [10][18].
However, according to the structural models of PARP1-bound to DNA (PBD 4DQY), [10][44] (Figure 2A,B), DNA breaks are assumed to prevent the binding of PARP1 to phosphorylated Erk since the binding sites of phosphorylated Erk in PARP1 bound to DNA are occluded [10][15]. Accordingly, Erk-induced PARP1 polyADP-ribosylation is not anticipated in the presence of damaged DNA [10][15] and downregulation of Erk-induced gene expression is anticipated [10]. These assumptions were supported by several findings.
References
- Langelier, M.-F.; Eisemann, T.; Riccio, A.A.; Pascal, J.M. PARP family enzymes:regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 2018, 53, 187–198.
- Kallberg, T.; Langelier, M.-F.; Pascal, J.; Schuler, H. Structural biology of writers, readers, and erasers in mono- and poly(ADP-ribose)mediated signaling. Mol. Asp. Med. 2013, 34, 1089–1108.
- Dantzer, F.; Amé, J.C.; Schreiber, V.; Nakamura, J.; Ménissier-de Murcia, J.; de Murcia, G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006, 409, 493–510.
- Luscher, B.; Butepage, M.; Eckei, L.; Kreig, S.; Verheugd, P. ADP ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem. Rev. 2018, 118, 1092–1136.
- Langelier, M.F.; Planck, J.L.; Roy, S.; Pascal, J.M. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science 2012, 336, 728–732.
- Poirier, G.G.; deMurcia, G.; Jongstra-Bilen, J.; Mandel, P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc. Natl. Acad. Sci. USA 1982, 79, 3423–3427.
- Tulin, A.; Spradling, A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puf loci. Science 2003, 299, 560–562.
- Kraus, W.L.; Lis, J.T. PARP goes transcription. Cell 2003, 113, 677–683.
- Krishnakumar, R.; Gamble, M.J.; Frizzell, K.M.; Berocal, J.G.; Kininis, M.; Kraus, W.L. Reciprocal binding of PARP-1 and histone H1 at promoters specifes transcriptional outcomes. Science 2008, 319, 819–821.
- Visochek, L.; Grigoryan, G.; Kalal, A.; Milshtein-Parush, H.; Gazit, N.; Slutsky, I.; Yeheskel, A.; Shainberg, A.; Castiel, A.; Seger, R. A PARP1-ERK2 synergism is required for the induction of LTP L. Sci. Rep. (Nat.) 2016, 6, 24950.
- Izzo, A.; Schneider, R. The role of linker histone H1modifications in the regulation of gene expression and chromatin dynamics. Biochim. Biophys. Acta 2016, 1859, 486–495.
- Azad, G.K.; Ito, K.; Sailaja, T.; Biran, A.; Nissim-Rafinia, M.; Yamada, Y.; Brown, D.T.; Takizawa, T.; Meshorer, E. PARP1-dependent eviction of the linker histone H1mediates immediate early gene expression during neuronal activation. J. Cell. Biol. 2017, 217, 473–481.
- Fontan-Lozano, A.; Suarez-Pereira, I.; Horillo, A.; del-Pozo-Martin, Y.; Hmadch, A. Histone H1 Poly-Ribosylation Regulates the Chromatin Alterations Required for Learning Consolidation. J. Neurosci. 2010, 30, 13305–13313.
- Cohen-Armon, M.; Yeheskel, A.; Pascal, J.M. Signal-induced PARP1-Erk synergism mediates IEG expression. Signal Transduct. Target. Ther. 2019, 4, 8.
- Cohen-Armon, M.; Visochek, L.; Rozensal, D.; Kalal, A.; Geistrikh, I.; Klein, R.; Bendetz-Nezer, S.; Yao, Z.; Seger, R. DNA-Independent PARP-1 Activation by Phosphorylated ERK2 Increases Elk1 Activity: A Link to Histone Acetylation. Mol. Cell 2007, 25, 297–308.
- Visochek, l.; Steingart, R.; Vulin-Shultzman, I.; Klein, R.; Priel, E.; Gozes, I.; Cohen-Armon, M. PolyADP-ribosylation is involved in neurotrophic activity. J. Neurosci. 2005, 25, 7420–7428.
- Cohen-Armon, M. PARP-1 activation in the ERK signaling pathway. Trends Pharmacol. Sci. 2007, 28, 556–560.
- Visochek, L.; Cohen-Armon, M. PARP1-Erk synergism in proliferating cells. Oncotarget 2018, 9, 29140–29145.
- Berridge, M.J.; Irvine, R.F. Inositol phosphates and cell signaling. Nature 1989, 341, 197–204.
- Banno, Y.; Nagao, S.; Katada, T.; Nagata, K.; Ui, M.; Nozawa, Y. Stimulation by GTP-binding proteins (Gi, Go) of partially purified phospholipase C activity from human platelet membranes. Biochem. Biophys. Res. Commun. 1987, 146, 861–869.
- Exton, J.H. Signaling through phosphatidylcholine breakdown. J. Biol. Chem. 1990, 265, 1–4.
- Hennager, D.J.; Welsh, M.J.; Delise, S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-triphosphate levels can control nuclear Ca+2 concentration. J. Biol. Chem. 1995, 270, 4959–4962.
- Malviya, A.N.; Rogue, P.J. “Tell me where is calcium bred”: Clarifying the roles of nuclear calcium. Cell 1998, 92, 17–23.
- Gerasimenko, O.V.; Gerasimenko, V.J.; Tepikin, A.V.; Petersen, O.H. ATP-dependent accumulation and inositol trisphos phateor cyclic ADPribose-mediated release of Ca+2 from the nuclear envelope. Cell 1995, 80, 439–444.
- Cohen-Armon, M. PARP1 activation is required for long-term memory. In Long-Term Memory: Mechanisms, Types and Disorders. Series: Neurosci. Res. Progress, Perspectives on Cognitive Psychology; Alexandrov, A.K., Fedoseev, L.M., Eds.; NOVA Sci. Pub.: New York, NY, USA, 2012.
- Homburg, S.; Visochek, L.; Moran, N.; Dantzer, F.; Priel, E.; Asculai, E.; Schwartz, D.; Rotter, V.; Dekel, N.; Cohen-Armon, M. A Fast Signal–Induced Activation of Poly(Adp-Ribose) Polymerase: A Novel Downstream Target of Phospholipase C. J. Cell Biol. 2000, 150, 293–308.
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thoronton, J.M. Metal ions in biological catalysis: From Enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–12018.
- Ju, B.-G.; Schum, D.; Song, E.J.; Lee, K.-J.; Ross, D.W.; Glass, C.K.; Rosenfeld, M.G. Activating the PARP1-sensor component of the Groucho/TLE1 core pressor complex mediates a CaMKinase dependent neurogenic gene activation pathway. Cell 2004, 119, 815–829.
- Payne, M.E.; Fong, Y.L.; Ono, T.; Collbran, R.J.; Kemp, B.E.; Shoderling, T.R.; Means, A.R. Calcium/calmodulin-dependent protein kinase II. J. Biol Chem. 1988, 263, 7190–7199.
- Kuo, P. The mechanism of protein kinase C activation. Trends Neurosci. 1989, 12, 425–432.
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18.
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase:Multiple substrates regulate cellular functions. Growth Factors 2006, 24, 21–44.
- Khokhlachev, A.V.; Canagarajah, B.; Wilsbacher, J.; Robinson, M.; Atkinson, M.; Goldsmith, E.; Cobb, M. Phosphorylation of MAP kinase Erk2 promotes its homodimerization and nuclear translocation. Cell 1998, 91, 605–615.
- Casar, B.; Pinto, A.; Crespo, P. ERK dimers and Scaffold proteins, Un-expected partners for a forgotten (cytoplasmic) task. Cell Cycle 2009, 8, 7–17.
- Bhalla, U.S.; Iyengar, R. Emergent properties of networks of biological signaling pathways. Science 1999, 283, 381–387.
- Sasagawa, S.; Ozaki, Y.-I.; Fujita, K.; Kuroda, S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat. Cell Biol. 2005, 7, 365–373.
- Buchwalter, G.; Gross, C.; Wasylyk, B. Ets ternary complex transcription factors. Gene 2004, 324, 1–14.
- Pouyssegur, J.; Lenormand, P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signaling. Eur. J. Biochem. 2003, 270, 3291–3299.
- Busca, R.; Pouyssegur, J.; Lenormand, P. ERK1 and ERK2 Map Kinases: Specific roles or functional redundancy. Front. Cell Dev. Biol. 2016, 4, 1–23.
- Geistrikh, I.; Visochek, L.; Klein, R.; Miller, L.; Mittelman, L.; Shainberg, A.; Cohen-Armon, M. Ca+2-induced PARP-1 activation and ANF expression are coupled events in cardiomyocytes. Biochem. J. 2011, 438, 337–347.
- Tanoue, T.; Adachi, M.; Moriguchi, T.; Nishida, E. A conserved docking motif in MAP kinases common to substrates activetors and regulators. Nat. Cell Biol. 2000, 2, 110–116.
- Jacobs, D.; Glossip, D.; Xing, H.; Muslin, A.J.; Kornfeld, K. Multiple docking sites on substrate proteins form modular system that mediates recognition by Erk MAP kinase. Gene Dev. 1999, 13, 163–175.
- Tanoue, T.; Nishida, E. Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol. Ther. 2002, 2–3, 193–202.
- Atilgan, A.R.; Durell, S.R.; Jernigan, R.L.; Demirel, M.C.; Keskin, O. Anisotropy of fluctuation dynamics of protein with elastic network model. Biophys. J. 2001, 80, 505–515.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




