
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Romualdo Sciorio | -- | 2469 | 2022-05-16 22:09:37 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2468 | 2022-05-17 09:54:15 | | |
Video Upload Options
Intracytoplasmic sperm injection (ICSI) has been used for severe male factor infertility and non-male factors, such as unexplained infertility or advanced maternal age, without robust scientific evidence. However, applying ICSI blindly is not free of potential detrimental consequences since novel studies report possible health consequences to offspring. DNA methylation and epigenetic alterations in sperm cells of infertile men might help explain some of the adverse effects reported in ICSI studies on reproductive health in future generations.
1. Background
2. Imprinting Alteration following ART
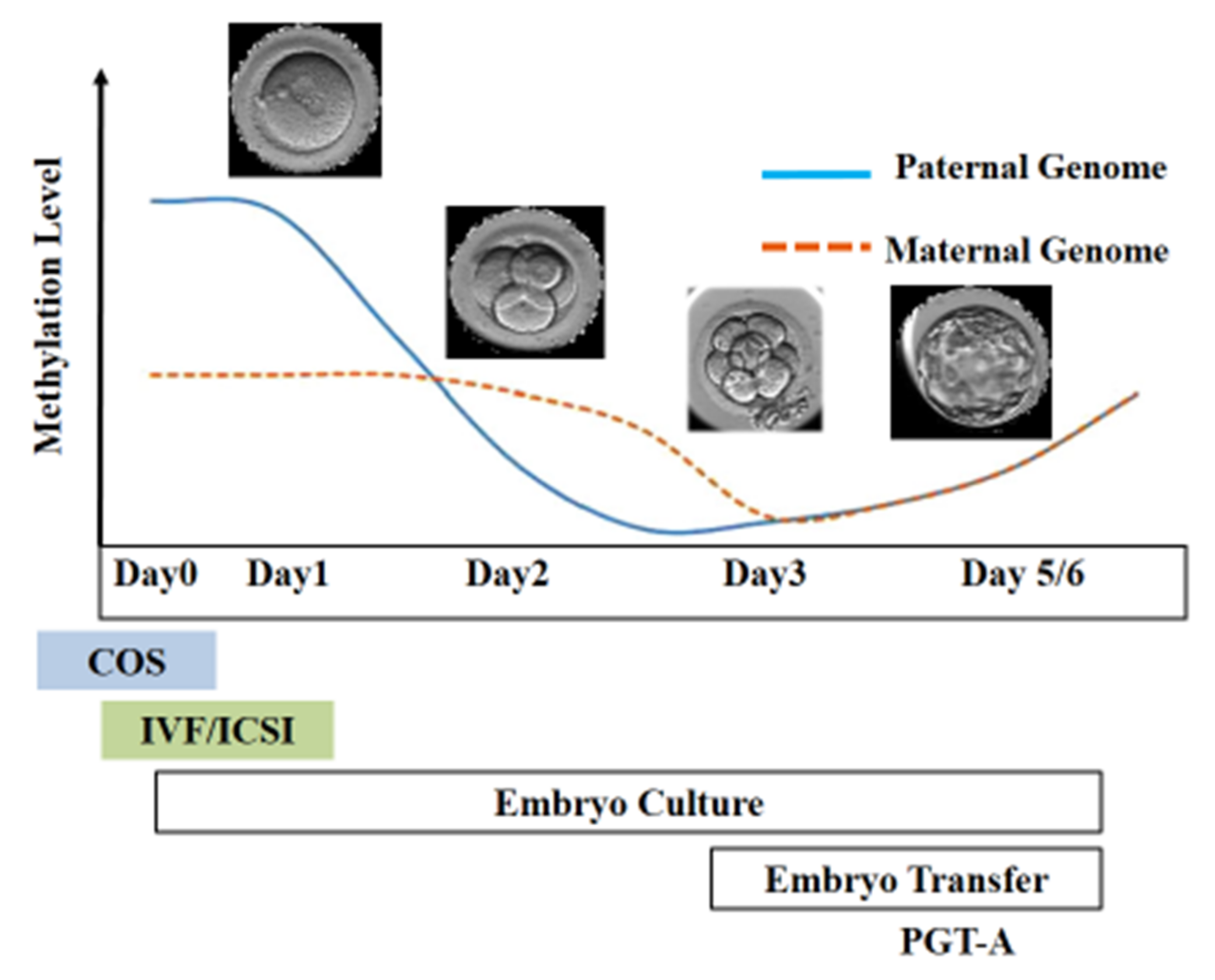
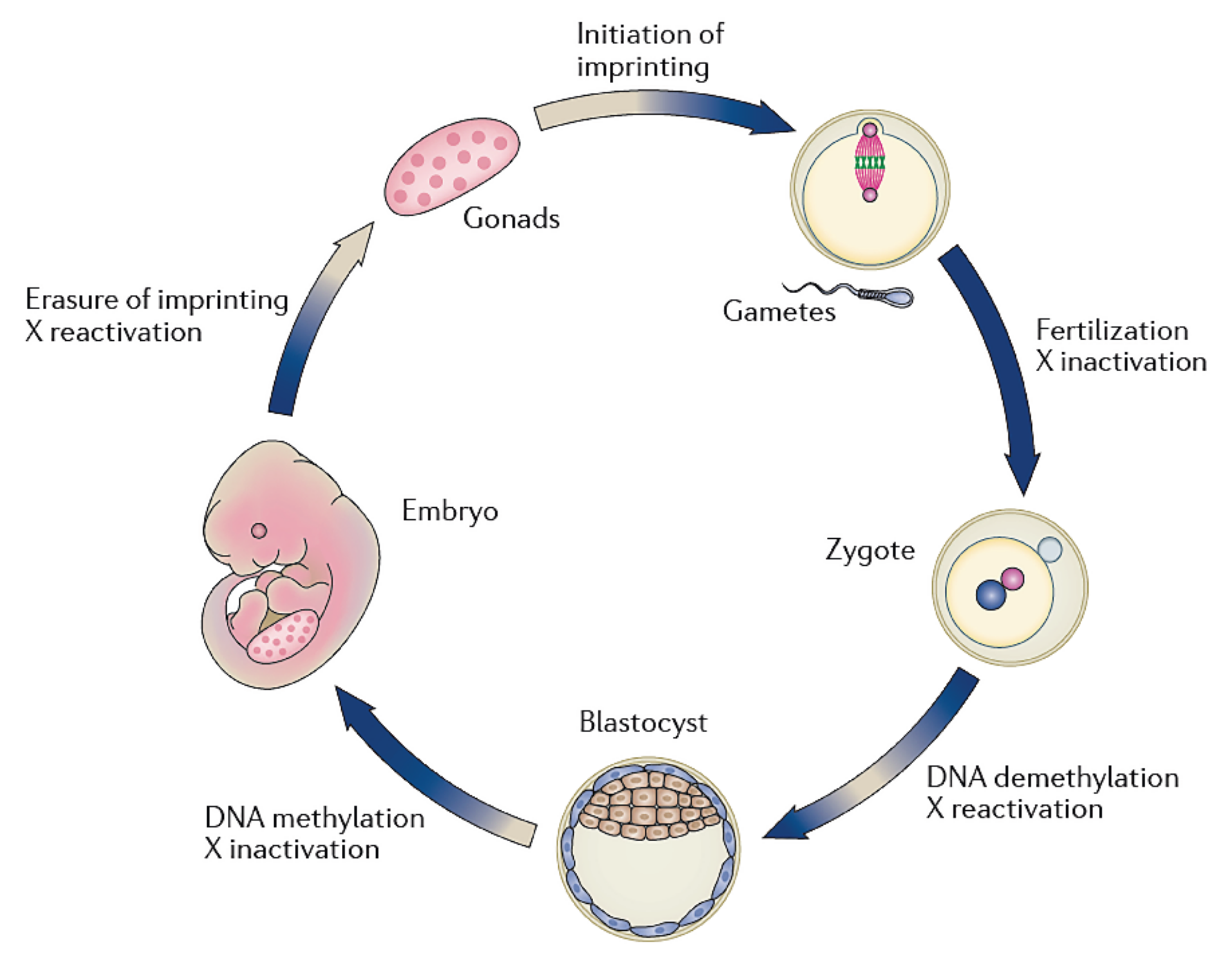
3. Spermatogenesis, Epigenetics, and Infertility
4. ICSI for Male Factor Infertility
4.1. Oligoasthenoteratozoospermia
4.2. Azoospermia
4.3. Antisperm Antibodies
4.4. ICSI and Sperm DNA Fragmentation (SDF)
4.5. Globozoospermia
5. Use of ICSI for Couples with Partners Having Semen Analysis within Reference Ranges
One of the first Cochrane paper was published in 2004 by van Rumste and collaborators to investigate whether ICSI improves LBR compared to IVF in couples whose male partners had semen analysis within reference ranges. The scholars showed a significantly higher fertilization rate in the IVF group but no difference in pregnancy, miscarriage, or LBR than ICSI insemination [55]. Subsequently, Bhattacharya and co-workers performed a multicenter randomized controlled study comparing clinical outcomes after ICSI or traditional IVF in couples with male partners having semen assessment within references ranges. The study randomly assigned 415 couples and was performed in four UK IVF units. Their results showed that the fertilization rate was higher with IVF than with ICSI (58% versus 47%; p = 0.0001). Standard IVF insemination provided an implantation rate of 30% compared to 22% for ICSI (p = 0.03). No significant difference was observed regarding the clinical pregnancy rate between IVF and ICSI (33% and 26%, respectively). Moreover, the overall laboratory time used was significantly shorter with IVF than with ICSI (22.9 min versus 38.1) [56]. Dang and co-workers reported similar results. They randomized 1064 patients undergoing ART to ICSI technique (n = 532) or standard IVF insemination (n = 532). After the first embryo transfer, LBR was 35% in the ICSI group versus 31% for couples assigned to conventional IVF (p = 0.27). They found higher TFF with IVF (6%) than with ICSI (5%). The study concluded that in couples undergoing ART with a male partner having so-called normal semen parameters, ICSI did not increase LBR compared with conventional IVF [57].
6. Contemporary Use (and Overuse) of ICSI
References
- De Geyter, C.; Wyns, C.; Calhaz-Jorge, C.; De Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Andersen, A.N.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849.
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 2, 366.
- Hiura, H.; Okae, H.; Chiba, H.; Miyauchi, N.; Sato, F.; Sato, A.; Arima, T. Imprinting methylation errors in ART. Reprod. Med. Biol. 2014, 13, 193–202.
- Ventura-Juncá, P.; Irarrázaval, I.; Rolle, A.J.; Gutiérrez, J.I.; Moreno, R.D.; Santos, M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015, 48, 68.
- Qin, J.; Sheng, X.; Wang, H.; Liang, D.; Tan, H.; Xia, J. Assisted reproductive technology and risk of congenital malformations: A metaanalysis based on cohort studies. Arch. Gynecol. Obstet. 2015, 292, 777–798.
- Hart, R.; Norman, R.J. The longer-term health outcomes for children born as a result of IVF treatment: Part I—General health outcomes. Hum. Reprod. Update 2013, 19, 232–243.
- Kessler, N.J.; Waterland, R.A.; Prentice, A.M.; Silver, M.J. Establishment of environmentally sensitive DNA methylation states in the very early human embryo. Sci. Adv. 2018, 4, 1–9.
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18.
- Palermo, G.D.; Neri, Q.V.; Rosenwaks, Z. To ICSI or not to ICSI. Semin. Reprod. Med. 2015, 33, 92–102.
- El Hajj, N.; Haertle, L.; Dittrich, M.; Denk, S.; Lehnen, H.; Hahn, T.; Schorsch, M.; Haaf, T. DNA methylation signatures in cord blood of ICSI children. Hum. Reprod. 2017, 32, 1761–1769.
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84.
- Gianotten, J.; Lombardi, M.P.; Zwinderman, A.H.; Lilford, R.J.; van der Veen, F. Idiopathic impaired spermatogenesis: Genetic epidemiology is unlikely to provide a short-cut to better understanding Hum. Reprod. Update 2004, 10, 533–539.
- Gunes, S.; Arslan, M.A.; Hekim, G.N.T.; Asci, R. The role of epigenetics in idiopathic male infertility. J. Assist. Reprod. Genet. 2016, 33, 553–569.
- Zhao, Y.; Garcia, B.A. Comprehensive catalog of currently documented histone modifications. Cold Spring Harb. Perspect. Biol. 2015, 7, a025064.
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705.
- Eggermann, T.; Perez de Nanclares, G.; Maher, E.R.; Temple, I.K.; Tümer, Z.; Monk, D.; Mackay, D.J.; Grønskov, K.; Riccio, A.; Linglart, A.; et al. Imprinting disorders: A group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin. Epigenet. 2015, 7, 123.
- White, C.R.; Denomme, M.M.; Tekpetey, F.R.; Feyles, V.; Power, S.G.; Mann, M.R. High frequency of imprinted methylation errors in human preimplantation embryos. Sci. Rep. 2015, 5, 17311.
- Huntriss, J.D.; Hemmings, K.E.; Hinkins, M.; Rutherford, A.J.; Sturmey, R.G.; Elder, K.; Picton, H.M. Variable imprinting of the MEST gene in human preimplantation embryos. Eur. J. Hum. Genet. 2013, 21, 40–47.
- Chen, Z.; Robbins, K.M.; Wells, K.D.; Rivera, R.M. Large offspring syndrome: A bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics 2013, 8, 591–601.
- Tunster, S.J.; Jensen, A.B.; John, R.M. Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 2013, 145, R117–R137.
- Hiura, H.; Okae, H.; Miyauchi, N.; Sato, F.; Sato, A.; Van De Pette, M.; John, R.M.; Kagami, M.; Nakai, K.; Soejima, H.; et al. Characterization of DNA methylation errors in patients with imprinting disorders conceived by assisted reproduction technologies. Hum. Reprod. 2012, 27, 2541–2548.
- Esteves, S.C.; Roque, M.; Bedoschi, G.; Haahr, T.; Humaidan, P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat. Rev. Urol. 2018, 15, 535–562.
- Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8.
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469.
- Leslie, M. Epigenetics. Sperm RNA fragments modify offspring metabolism. Science 2016, 351, 13.
- Ben Maamar, M.; Beck, D.; Nilsson, E.; McCarrey, J.R.; Skinner, M.K. Affiliations expand Developmental alterations in DNA methylation during gametogenesis from primordial germ cells to sperm. iScience 2022, 25, 103786.
- Cornwall, G.A. Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control. Adv. Exp. Med. Biol. 2014, 759, 159–180.
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698.
- Sullivan, R.; Legare, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757.
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377.
- Sharma, A. Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance. Mech. Ageing Dev. 2017, 163, 15–22.
- Scott, I.M.; Rubinstein, G.M.; Poole, F.L., II; Lipscomb, G.L.; Schut, G.J.; Williams-Rhaesa, A.M.; Stevenson, D.M.; Amador-Noguez, D.; Kelly, R.M.; Adams, M.W.W. The thermophilic biomass-degrading bacterium Caldicellulosiruptor bescii utilizes two enzymes to oxidize glyceraldehyde 3-phosphate during glycolysis. J. Biol. Chem. 2019, 294, 9995–10005.
- Ben Maamar, M.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; McCarrey, J.R.; Skinner, M.K. Developmental origins of transgenerational sperm DNA methylation epimutations following ancestral DDT exposure. Dev. Biol. 2019, 445, 280–293.
- Esteves, S.C.; Zini, A.; Aziz, N.; Alvarez, J.G.; Sabanegh, E.S., Jr.; Agarwal, A. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology 2012, 79, 16–22.
- Babayev, S.N.; Park, C.W.; Bukulmez, O. Intracytoplasmic sperm injection indications: How rigorous? Semin. Reprod. Med. 2014, 32, 283–290.
- Shuai, H.L.; Ye, Q.; Huang, Y.H.; Xie, B.G. Comparison of conventional in vitro fertilisation and intracytoplasmic sperm injection outcomes in patients with moderate oligoasthenozoospermia. Andrologia 2015, 47, 499–504.
- Kruger, T.F.; Menkveld, R.; Stander, F.S.; Lombard, C.J.; Van der Merwe, J.P.; van Zyl, J.A.; Smith, K. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil. Steril. 1986, 46, 1118–1123.
- Gunalp, S.; Onculoglu, C.; Gurgan, T.; Kruger, T.F.; Lombard, C.J. A study of semen parameters with emphasis on sperm morphology in a fertile population: An attempt to develop clinical thresholds. Hum. Reprod. 2001, 16, 110–114.
- Menkveld, R.; Wong, W.Y.; Lombard, C.J.; Wetzels, A.M.; Thomas, C.M.; Merkus, H.M.; Steegers-Theunissen, R.P. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An attempt to develop clinical thresholds. Hum. Reprod. 2001, 16, 1165–1171.
- Plachot, M.; Belaisch-Allart, J.; Mayenga, J.M.; Chouraqui, A.; Tesquier, L.; Serkine, A.M. Outcome of conventional IVF and ICSI on sibling oocytes in mild male fator infertility. Hum. Reprod. 2002, 17, 362–369.
- Devroey, P.; Van Steirteghem, A. A review of ten years experience of ICSI. Hum. Reprod. Update 2004, 10, 19–28.
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male . Clinics 2011, 66, 691–700.
- Esteves, S.C.; Miyaoka, R.; Orosz, J.E.; Agarwal, A. An update on sperm retrieval techniques for azoospermic males. Clinics 2013, 68 (Suppl. 1), 99–110.
- Esteves, S.C. Novel concepts in male factor infertility: Clinical and laboratory perspectives. J. Assist. Reprod. Genet. 2016, 33, 1319–1335.
- Zini, A.; Fahmy, N.; Belzile, E.; Ciampi, A.; Al-Hathal, N.; Kotb, A. Antisperm antibodies are not associated with pregnancy rates after IVF and ICSI: Systematic review and meta- analysis. Hum. Reprod. 2011, 26, 1288–1295.
- Esteves, S.C.; Schneider, D.T.; Verza, S., Jr. Influence of antisperm antibodies in the semen on intracytoplasmic sperm injection outcome. Int. Braz. J. Urol. 2007, 33, 795–802.
- Roque, M.; Esteves, S.C. Effect of varicocele repair on sperm DNA fragmentation: A review. Int. Urol. Nephrol. 2018, 50, 583–603.
- Esteves, S.C.; Sharma, R.K.; Gosálvez, J.; Agarwal, A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int. Urol. Nephrol. 2014, 46, 1037–1052.
- Greco, E.; Scarselli, F.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Franco, G.; Anniballo, N.; Mendoza, C.; Tesarik, J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum. Reprod. 2005, 20, 226–230.
- Majzoub, A.; Esteves, S.C.; Gosálvez, J.; Agarwal, A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J. Androl. 2016, 18, 205–212.
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620.
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia 2021, 53, e13874.
- Practice Committees of American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil. Steril. 2013, 99, 673–677.
- Dam, A.; Feenstra, I.; Westphal, J.; Ramos, L.; van Golde, R.; Kremer, J. Globozoospermia revisited. Hum. Reprod. Update 2007, 13, 63–75.
- Van Rumste, M.M.; Evers, J.L.; Farquhar, C.M. ICSI versus conventional techniques for oocyte insemination during IVF in patients with non- male factor subfertility a Cochrane review. Hum. Reprod. 2004, 19, 223–227.
- Bhattacharya, S.; Hamilton, M.; Shaaban, M.; Khalaf, Y.; Seddler, M.; Ghobara, T.; Braude, P.; Kennedy, R.; Rutherford, A.; Hartshorne, G.; et al. Conventional in- vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non- male-factor infertility: A randomised controlled trial. Lancet 2001, 357, 2075–2079.
- Dang, V.Q.; Vuong, L.N.; Luu, T.M.; Pham, T.D.; Ho, T.M.; Ha, A.N.; Truong, B.T.; Phan, A.K.; Nguyen, D.P.; Pham, T.N.; et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation in couples with infertility in whom the male partner has normal total sperm count and motility: An open-label, randomised controlled trial. Lancet 2021, 397, 1554–1563.
- Jain, T.; Gupta, R. Trends in the use of intracytoplasmic sperm injection in the United States. N. Engl. J. Med. 2007, 357, 251–257.
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted reproductive technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609.
- Cissen, M.; Bensdorp, A.; Cohlen, B.J.; Repping, S.; de Bruin, J.P.; van Wely, M. Assisted reproduction technologies for male subfertility. Cochrane Database Syst. Rev. 2016, 2, CD000360.
- Boulet, S.L.; Mehta, A.; Kissin, D.M.; Warner, L.; Kawwass, J.F.; Jamieson, J.D. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015, 313, 255–263.
- Chambers, G.M.; Wand, H.; Macaldowie, A.; Chapman, M.G.; Farquhar, C.M.; Bowman, M.; Molloy, D.; Ledger, W. Population trends and live birth rates associated with common ART treatment strategies. Hum. Reprod. 2016, 31, 2632–2641.
- Practice Committees of American Society for Reproductive Medicine and Society for Assisted Reproduction Technology. Intracytoplasmic sperm injection (ICSI) for non- male factor indications: A committee opinion. Fertil. Steril. 2020, 114, 239–245.




