Intracytoplasmic sperm injection (ICSI) has been used for severe male factor infertility and non-male factors, such as unexplained infertility or advanced maternal age, without robust scientific evidence. However, applying ICSI blindly is not free of potential detrimental consequences since novel studies report possible health consequences to offspring. DNA methylation and epigenetic alterations in sperm cells of infertile men might help explain some of the adverse effects reported in ICSI studies on reproductive health in future generations.
- assisted reproductive technology
- human in vitro fertilization
- embryo development
- male infertility
- intracytoplasmic sperm injection
1. Background
2. Imprinting Alteration following ART
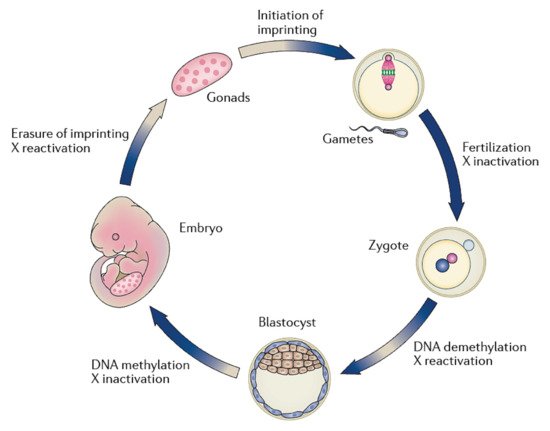
After fertilization, the zygote develops into a structure called the “blastocyst”, which includes about 200 cells, already differentiated into two types: the trophectoderm (TE) and the inner cell mass (ICM). The latter comprises a group of cells attached to the inside of the trophectoderm, which will eventually give rise to the fetus. TE cells are the blastocyst’s external layer, promoting the implantation process into the uterine lining and forming other extraembryonic tissues, including the placenta. Embryonic cells are guided toward their future lineages during early development through epigenetic reprogramming and subsequent re-establishment of cell-type-specific epigenetic signatures. This coincides with the period when gametes and embryos are cultivated inside the embryology laboratory. Therefore, during this critical time window, any artificial perturbations might lead to epigenetic modifications in the resultant offspring (Figure 1 and Figure 2). Studies reported imprinted loci to be vulnerable to external environmental cues during in vitro embryo culture. For example, KvDMR1 has been abnormally methylated in ART-related BWS in humans [16][17][18][42,60,61] and hypomethylated in ART-produced bovine progeny with large offspring syndrome (LOS) [19][62]. Additionally, several reports indicate that ART-related procedures, including OS, ICSI, and extended culture to the blastocyst stage, might promote epigenetic aberrations [16][20][21][41,42,55].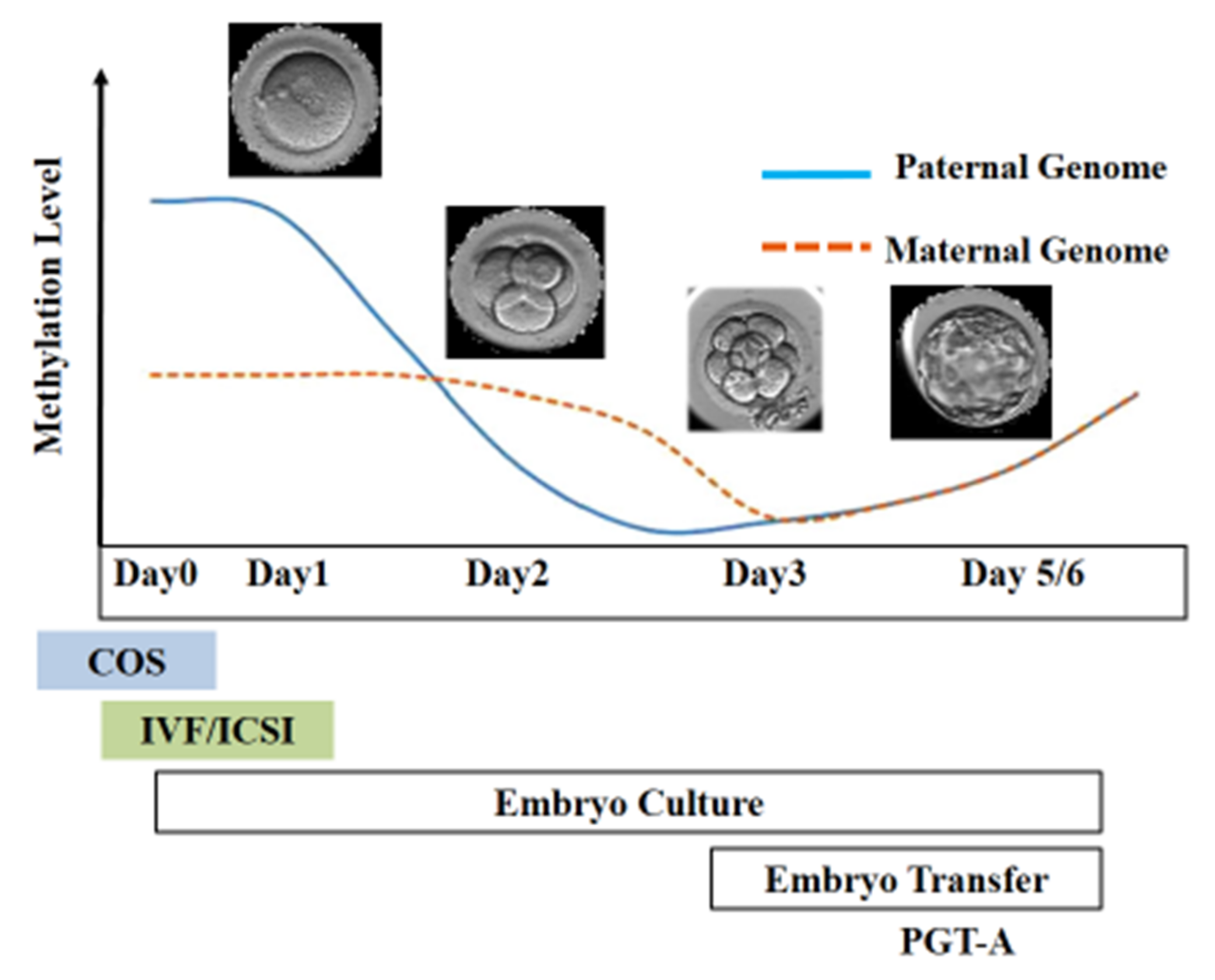
3. Spermatogenesis, Epigenetics, and Infertility
45. ICSI for Male Factor Infertility
4.1. Oligoasthenoteratozoospermia
5.1. Oligoasthenoteratozoospermia
Although ICSI should be encouraged mainly in severe male infertility, it can be challenging to establish when a male factor is compulsory for the ICSI technique. Standard semen assessment is performed to confirm the severity of male infertility and advise ICSI, but it is well reported that sperm analysis has limitations; for example, it does not assess the function and physiology of the sperm, and genetic or epigenetic assessment [34][83]. Sperm number, morphology, and motility are typically evaluated to decide on the ICSI procedure rather than standard IVF insemination [35][84]. It is worth mentioning that high-quality studies investigating pregnancy outcomes and live birth rate (LBR) between ICSI and IVF in couples with oligoasthenoteratozoospermia are still missing. However, a study published in 2005 by Shuai and collaborators explored these concerns. The scholarauthors observed no differences between the two insemination procedures (IVF and ICSI) in fertilization, implantation, and pregnancy rates in couples undergoing ART with men diagnosed with moderate oligoasthenoteratozoospermia [36][85]. Sperm morphology is another parameter broadly used to choose for ICSI. In 1986, Kruger and colleagues suggested using strict criteria for sperm abnormalities and advising ICSI when the proportion of normal sperm in the ejaculate was <4% [37][86]. Additional studies confirmed this evidence and proposed that at least 5% of sperm is needed to be morphologically normal to obtain an acceptable fertilization rate using standard IVF [38][39][87,88]. Therefore, ICSI rather than IVF has been routinely recommended in patients with reduced sperm morphology (<5%) [40][89].4.2. Azoospermia
5.2. Azoospermia
The term azoospermia indicates the absence of sperm cells in the ejaculate. It affects around 1% of the general male population and about 15% of infertile men [41][93]. There are two different types of azoospermia: obstructive and non-obstructive. In obstructive azoospermia, normal and complete spermatogenesis is typically found, and sperm can be surgically collected from the testis [42][94]. By contrast, non-obstructive azoospermia is associated with the testicular alterations that result in the failure of sperm production. Typical testicular histopathological features in males with non-obstructive azoospermia include germ cell aplasia, maturation arrest, or hypospermatogenesis. The procedures mostly applied to collect sperm from azoospermic patients are percutaneous acquisition and open surgery [43][95]. Following sperm retrieval, ICSI can be applied to achieve oocyte fertilization [44][96].4.3. Antisperm Antibodies
5.3. Antisperm Antibodies
The presence of seminal antisperm antibodies (ASAs) is typically associated with a gap or rupture of the blood–testis barrier in the reproductive tract, which can be linked with several conditions [45][97]. However, elevated levels of ASAs in semen samples are observed in about 5–12% of men undergoing ART, and might negatively affect fertility, reducing sperm motility, capacitation, acrosome reaction, and oocyte sperm bounding [46][98].4.4. ICSI and Sperm DNA Fragmentation (SDF)
5.4. ICSI and Sperm DNA Fragmentation (SDF)
DNA fragmentation test is applied to assess the breakage of DNA strands inside the sperm head. This diagnostic test can predict fertility and normal embryo development and pregnancy outcomes than routine semen analysis parameters [47][48][99,100]. With the use of probes, sperm DNA breaks can be deeply scrutinized and quantified with the aid of fluorescence/optical microscopy or flow cytometry [48][100]. Sperm DNA fragmentation (SDF) is generally induced by oxidative stress resulting from environmental and lifestyle factors such as smoking, genital tract infections, obesity, and nutrition [49][101]. Moreover, SDF is frequently detected in men with infertility issues (e.g., varicocele), and it is more prevalent in those individuals than in fertile counterparts [50][51][102,103]. Scientific evidence indicates that a high level of SDF impairs the probabilities of success following ART [52][53][104,105].4.5. Globozoospermia
5.5. Globozoospermia
This condition is described by the entire lack of the acrosomal vesicle in the sperm head, with alteration of the nuclear membrane, and midpiece defects, resulting in a round-shaped sperm head. It is an uncommon condition involving a small percentage of infertile men (about 0.1%) [54][111]. Despite having normal sperm count and motility, globozoospermic sperm cannot fertilize the oocyte: therefore, ICSI remains the favorable option available.56. Use of ICSI for Couples with Partners Having Semen Analysis within Reference Ranges
One of the first Cochrane review papers was published in 2004 by van Rumste and collaborators to investigate whether ICSI improves LBR compared to IVF in couples whose male partners had semen analysis within reference ranges. The scholarauthors showed a significantly higher fertilization rate in the IVF group but no difference in pregnancy, miscarriage, or LBR than ICSI insemination [55][113]. Subsequently, Bhattacharya and co-workers performed a multicenter randomized controlled study comparing clinical outcomes after ICSI or traditional IVF in couples with male partners having semen assessment within references ranges. The study randomly assigned 415 couples and was performed in four UK IVF units. Their results showed that the fertilization rate was higher with IVF than with ICSI (58% versus 47%; p = 0.0001). Standard IVF insemination provided an implantation rate of 30% compared to 22% for ICSI (p = 0.03). No significant difference was observed regarding the clinical pregnancy rate between IVF and ICSI (33% and 26%, respectively). Moreover, the overall laboratory time used was significantly shorter with IVF than with ICSI (22.9 min versus 38.1) [56][114]. Dang and co-workers reported similar results. They randomized 1064 patients undergoing ART to ICSI technique (n = 532) or standard IVF insemination (n = 532). After the first embryo transfer, LBR was 35% in the ICSI group versus 31% for couples assigned to conventional IVF (p = 0.27). They found higher TFF with IVF (6%) than with ICSI (5%). The study concluded that in couples undergoing ART with a male partner having so-called normal semen parameters, ICSI did not increase LBR compared with conventional IVF [57][115].
