Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Teresa Tellez | -- | 1968 | 2022-05-15 10:03:09 | | | |
| 2 | Camila Xu | Meta information modification | 1968 | 2022-05-16 03:23:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tellez, T.; , .; Redondo, M.; García-Aranda, M. The Role of Tachykinins in Human Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/22947 (accessed on 08 February 2026).
Tellez T, , Redondo M, García-Aranda M. The Role of Tachykinins in Human Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/22947. Accessed February 08, 2026.
Tellez, Teresa, , Maximino Redondo, Marilina García-Aranda. "The Role of Tachykinins in Human Disease" Encyclopedia, https://encyclopedia.pub/entry/22947 (accessed February 08, 2026).
Tellez, T., , ., Redondo, M., & García-Aranda, M. (2022, May 15). The Role of Tachykinins in Human Disease. In Encyclopedia. https://encyclopedia.pub/entry/22947
Tellez, Teresa, et al. "The Role of Tachykinins in Human Disease." Encyclopedia. Web. 15 May, 2022.
Copy Citation
Since the identification of substance P in 1931, a number of short, highly conserved, bioactive peptides, called tachykinins, have been isolated and investigated, constituting at present one of the largest families of neuropeptides. In humans, tachykinins are expressed throughout the nervous and immune system, with an important role in the regulation of a wide range of physiological processes that include inflammation, nociception, smooth muscle contractility, epithelial secretion and cell proliferation in the nervous, immune, gastrointestinal, respiratory, urogenital and dermal systems.
cancer
tachykinin
tachykinin receptor
NK-1R
targeted treatment
human
disease
1. The Challenge of Drug-Resistant Tumors
Cancer remains a major public health problem and a leading cause of death worldwide, accounting for more than 19 million new cases and nearly 10 million deaths in 2020 [1]. At the present time, one of the main challenges in the treatment of cancer patients is overcoming resistance, a clinical situation in which the tumor does not respond to treatment (intrinsic or primary resistance) or, although it initially responded, it ends up relapsing and progressing (secondary or acquired resistance).
Tumor progression is characterized by the sequential appearance of genetically altered cell subpopulations, which, under the selective pressure caused by anti-cancer treatments, promote the accumulation of irreversible changes and the proliferation of cell populations resistant to anticancer treatments [2]. This loss of therapeutic response of cancer cells against anti-cancer drugs, or multidrug resistance, can occur during or after treatment and may lead to the development of cross resistance to either structurally or mechanistically different chemotherapeutics.
The causes of multidrug resistance can include mechanisms as diverse as the elevated metabolism of xenobiotics, enhanced efflux of drugs, increased DNA repair capacity, genomic instability, reduced apoptosis or altered proliferation [3][4], which are, to some extent, related to the different hallmarks of cancer, as defined by Hanahan and Weinberg [5] (Figure 1). Nowadays, intrinsic and acquired multidrug resistance represent the main cause of treatment failure for patients with blood or solid tumors, being responsible for over 90% of deaths in oncologic patients treated with conventional chemotherapy or novel targeted treatments [3].
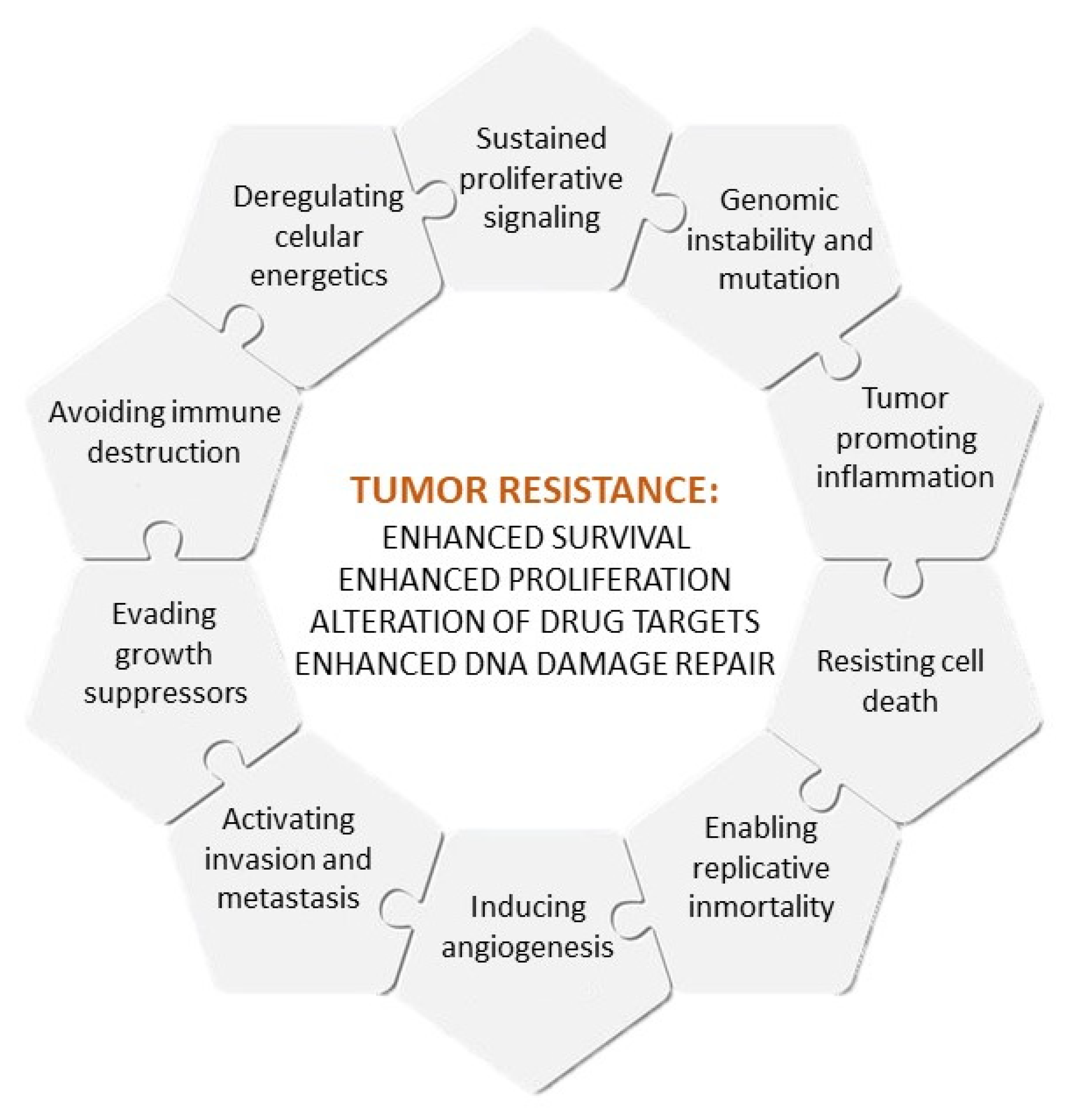 Figure 1. Hallmarks of cancer and causes of multidrug resistance.
Figure 1. Hallmarks of cancer and causes of multidrug resistance.Although recent advances in cancer research have allowed for the development of targeted treatments for resistant tumors, most of them have failed in clinical stages, mainly due to their low specificity and high toxicity [6]. For this reason, cancer research is nowadays focused on the development of more effective and safe targeted therapies.
As for other drugs, the development of new therapeutic agents for cancer treatment requires the validation of drug safety and efficacy, which involves multiple phases, from the earliest basic investigation to clinical testing and final authorization. Since this long-term process of authorization of new drugs for cancer treatment, from bench to bedside, is associated with a high risk of failure, timeframe and overall costs [7], the use of known marketed drugs, which have already been assessed for safety and efficacy, for new therapeutic purposes, or drug repurposing, has gained increasing popularity in recent times as a strategy to enhance new pharmaceutical development with rapid clinical translation [8], having proved to shorten the development process by 3–5 years and to increase the success rates from 10% to 25% [7]. So much so that up to 30% of all the current drugs and vaccines approved in recent years by the US Food and Drug Administration (FDA) are repurposed drugs [9], some of which are already being tested to see if they can be useful for the treatment of resistant tumors.
G-protein-coupled receptors (GPCRs) are the largest family proteins targeted by approved drugs, accounting for approximately 17% of FDA agents against human proteins [9][10]. In 2003, aprepitant (EMEND, Merck Sharp & Dohme B.V.) became the first tachykinin-1 receptor (TAC1R, aka neurokinin-1 receptor NK-1R) antagonist approved for the prevention of acute and delayed chemotherapy-induced nausea and vomiting, a side-effect that can affect more than 99% of patients treated with cisplatin [11].
Studies on cancer research also reported that both NK-1R and its preferred ligand (neurokinin 1, NK-1 aka substance P, SP) are overexpressed in a wide variety of malignancies, including leukemia, glioblastoma, astrocytoma, neuroblastoma, melanoma, breast, ovarian, prostate, lung, pancreas and thyroid cancer [12][13], with a role in different driving agents to resistance, such as angiogenesis, cancer cell proliferation, migration and metastasis [12][13][14][15][16]. As a result of a recent clinical report [17] and different in vitro and in vivo studies showing that NK-1R antagonists can exert an antitumor, antiproliferative, anti-survival, antiangiogenic and antimetastatic effect [14][16][18], the inhibition of the NK1-R/SP axis has been proposed as a promising therapeutic approach to battle cancer and cancer resistance [19][20][21], justifying additional investigations that support the reprofiling of marketed NK-1R antagonists, such as aprepitant, as therapeutic agents for cancer treatment, in addition to their use in clinical practice as antiemetic.
2. Tachykinins
Since the identification of substance P in 1931, a number of short, highly conserved, bioactive peptides, called tachykinins, have been isolated and investigated, constituting at present one of the largest families of neuropeptides. In humans, tachykinins are expressed throughout the nervous and immune system, with an important role in the regulation of a wide range of physiological processes that include inflammation, nociception, smooth muscle contractility, epithelial secretion and cell proliferation in the nervous, immune, gastrointestinal, respiratory, urogenital and dermal systems [22].
All members of this family are structurally related peptides characterized by an amidated C-terminal, whose deamidation suppresses peptide activity [23], and a conserved C-terminal sequence -Phe-Xaa-Gly-Leu-Met-NH2, where Xaa represents a hydrophobic amino acid residue [24][25] (Table 1) required for the activation of the corresponding receptor (tachykinin receptor 1/NK-1R, 2/NK-2R or 3/NK-3R) [22], which is responsible for signal transmission from the extracellular environment to the cytoplasm.
Table 1. Main members of the human tachykinin family.
| Gene | Tachykinin | Sequence | Preferred Tachykinin Receptor |
|---|---|---|---|
| TAC1 | Neurokinin 1 (NK1),Substance P (SP) | RPKPQQFFGLM [26] | Neurokinin 1 receptor (NK-1R) |
| Neurokinin A (NKA) Substance K (SK) | HKTDSFVGLM [26] | Neurokinin 2 receptor (NK-2R) | |
| Neuropeptide K (NPK) | DADSSIEKQVALLKALYGHGQISHKRHKTDSFVGLM [26] | Neurokinin 2 receptor (NK-2R) | |
| Neuropeptide γ (NP γ) | MKILVALAVFFLVSTQLFAEEIGANDDLNYWSDWYDSDQIKEELPEPFEHLLQRARRPKPQQFFGLMGKRDADSSIEKQVALLKALYGHGQISHKRHKTDSFVGLMGKRALNSVAYERSAMQNYERRR (1st part)GHGQISHKRHKTDSFVGLM (2nd part) [26] | Neurokinin 2 receptor (NK-2R) | |
| TAC3 | Neurokinin B (NKB)Neuromedin-K | DMHDFFVGLM [27] | Neurokinin 3 receptor (NK-3R) |
| TAC4 | Endokinin A (EKA) | DGGEEQTLSTEAETWVIVALEEGAGPSIQLQLQEVKTGKASQFFGLM [28] | Neurokinin 1 receptor (NK-1R) |
| Endokinin A/B (EKA/B) | GKASQFFGLM [28] | Neurokinin 1 receptor (NK-1R) | |
| Endokinin C (EKC) | KKAYQLEHTFQGLL [28] | Neurokinin 1 receptor (NK-1R) | |
| Endokinin D (EKD) | VGAYQLEHTFQGLL | Neurokinin 1 receptor (NK-1R) |
In humans, tachykinins are encoded by three different genes, TAC1 (tachykinin precursor 1), TAC3 (tachykinin precursor 3) and TAC4 (tachykinin precursor 4) (Table 1):
-
The transcription of the TAC1 gene (NCBI Gene ID: 6863) produces the pre-protachykinin-A (PPTA)-mRNA, which is converted into one of four splice variants coding for a pro-tachykinin polypeptide that contains NK-1 [29], Neurokinin A (NKA, formerly known as substance K) and the NH2-terminally extended forms of NAK neuropeptide K (NPK) and neuropeptide gamma (NPγ) [22][26][30]. These peptides function as neurotransmitters by interacting with nerve receptors and smooth muscle cells [30].
-
TAC3 (NCBI Gene ID: 6866) encodes a preprotein that is further cleaved to generate a mature secreted neuropeptide (neurokinin B, NKB). NKB is primarily expressed in the central and peripheral nervous systems and functions as a neurotransmitter [31]. NKB is a critical central regulator of gonadal function and its alterations are mainly associated with hypogonadotropic hypogonadism [32].
-
Finally, TAC4 (NCBI Gene ID: 255061) produces endokinins (EK) A, A/B, C and D as well as hemokinins [12][28], which are associated with the hematopoietic system and lymphocyte B maturation [12]. TAC4 gene products are thought to regulate different peripheral endocrine and paracrine functions, including blood pressure, the immune system and endocrine gland secretion [33].
3. Tachykinins and Tachykinin Receptors in Human Disease and as Pharmacological Targets
Tachykinins have been associated with different pathological processes, including neurogenic diseases, such as schizophrenia, Alzheimer’s disease and Huntington’s disease [34], as well as acute and chronic inflammation and pain, fibrosis, affective and addictive disorders, functional disorders of the intestine and urinary bladder, infection or cancer [22]. Since tachykinin signaling on targeted cells is mediated by tachykinin receptors, most therapeutic approaches are designed to block ligand–receptor interactions.
3.1. Tachykinins and Tachykinin Receptors in Human Disease
-
Respiratory disorders
Both SP and NKA can be released from airway nerves after noxious stimulation [35], and have a role in respiratory functions, such as the regulation of airway smooth muscle tone, vascular tone and mucus secretion, and immune functions [35]. The overexpression of NK-1R and NK-2R receptors is usually found in inflammatory airway diseases, such as bronchial asthma or chronic obstructive pulmonary disease [35][36], with a role in bronchoconstriction, airway hyperresponsiveness and airway inflammation caused by allergic and nonallergic stimuli, which has prompted the development of both selective and dual-selective NK-1R/NK-2R antagonists that have entered clinic studies with promising results [36][37].
-
Smooth muscle disfunction:
NK-2R is predominantly expressed on smooth muscle, with a role in the contraction of the intestinal, genito-urinary and respiratory tracts. The NK-2R antagonist MEN11420 (nepadutant) has demonstrated, both in preclinical and clinical studies, to be a well-tolerated and a potent, selective and competitive NK-2R inhibitor with an effective and long-lasting anti-spasmogenic effect without affecting basal gastrointestinal motility [38].
-
Central nervous system disorders:
Non-peptide antagonists of NK-3R, which is mainly expressed in the central nervous system, have already shown in preclinical studies their potential utility as a strategy for the treatment of central nervous system disorders [39], such as schizophrenia, major depressive disorder, panic attacks and Parkinson’s disease [39].
Given the role of the SP/NK-1R axis in neuroinflammation associated with different bacterial, viral, parasitic and neurodegenerative diseases of the central nervous system, NK-1R antagonists have recently been proposed as a promising therapeutic agent for the treatment of neuroinflammation [40].
-
Hormonal disorders:
Inactivating mutations in both NKB or its preferred receptor NK-3R have been associated with low gonadotropin-releasing hormone (GnRH) pulse frequency [41]. Both preclinical and clinical studies (phase I clinical trial EUDRACT 2013-004314-17) have shown that the oral administration of the NK-3R antagonist ESN364 is well-tolerated and selectively modulates gonadotropin secretion, which may be of help in the treatment of women’s health disorders with a low risk of menopausal-like adverse events [41].
3.2. Marketed Tachykinin Receptor Antagonists
SP roles in both health and disease have motivated intense research by the pharmaceutical industry to develop selective tachykinin receptor agonists that could be of help in the treatment of human disorders.
Concretely, SP is associated with physiological processes as diverse as hematopoiesis, wound healing, microvasculature permeability, neurogenic inflammation, leukocyte trafficking, cell survival and metastatic dissemination [12], as well as with the regulation of biological processes, such as the dilatation of the arterial vascular system, neuronal survival and degeneration, respiratory function, sensory perception, movement control of gastric motility, salivation, micturition, pain and depression [13].
On March 2006, the FDA approved EMEND® (aprepitant) as the first NK-1R antagonist for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly and moderately (approved on 28 October 2008) emetogenic cancer chemotherapy, as well as for the prevention of post-operative nausea and vomiting (approval on 30 June 2006) in adult patients [42].
Contrary to other previously developed drugs, aprepitant also included a novel nanoparticle formulation to optimize oral absorption that allows for its administration as a water-soluble phosphoryl prodrug form (Ivemend, fosaprepitant) suitable for intravenous administration [43]. Aprepitant selectively blocks the activation of NK-1R in vomiting centers within the central nervous system; thus, since approximately 25% and 50% of patients experience acute or delayed chemotherapy-induced nausea and vomiting, respectively [11], the marketing authorization of this non-peptide NK-1R antagonist represented a great step forward in enhancing the quality of life of oncologic patients who must undergo multiple cycles of chemotherapy [43].
In 2014, the US FDA approved the combination of NK-1R antagonist netupitant plus the 5-HT3R receptor antagonist palonosetron (Akynzeo; Eisai) for the prevention of acute and delayed nausea and vomiting associated with initial and repeated courses of cancer chemotherapy [44].
More recently, in 2015, the FDA also approved aprepitant for patients of 6 months of age and older [42] as well as Varubi© (rolapitant) tablets [45] and rolapitant injectable emulsion [46], as other non-peptide SP/NK-1R antagonists with the same indication as aprepitant/fosaprepitant, but with a better safety profile and lower incidence of adverse events [47].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- García-Aranda, M.; Pérez-Ruiz, E.; Redondo, M. Bcl-2 inhibition to overcome resistance to chemo-and immunotherapy. Int. J. Mol. Sci. 2018, 19, 3950.
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019, 9, 487.
- Frantzi, M.; Latosinska, A.; Mokou, M.; Mischak, H.; Vlahou, A. Drug repurposing in oncology. Lancet Oncol. 2020, 21, e543.
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113.
- Pillaiyar, T.; Meenakshisundaram, S.; Manickam, M.; Sankaranarayanan, M. A medicinal chemistry perspective of drug repositioning: Recent advances and challenges in drug discovery. Eur. J. Med. Chem. 2020, 195, 112275.
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258.
- European Medicines Agency. EMEND, INN-Aprepitant: Scientific Discussion. 2004. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/emend-epar-scientific-discussion_en.pdf (accessed on 28 May 2021).
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015, 2015, 495704.
- Rosso, M.; Munoz, M.; Berger, M. The role of neurokinin-1 receptor in the microenvironment of inflammation and cancer. Sci. World J. 2012, 2012, 381434.
- Muñoz, M.; Rosso, M.; Coveñas, R. A new frontier in the treatment of cancer: NK-1 receptor antagonists. Curr. Med. Chem. 2010, 17, 504–516.
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: A new approach. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 260–268.
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682.
- Muñoz, M.; Crespo, J.C.; Crespo, J.P.; Coveñas, R. Neurokinin-1 receptor antagonist aprepitant and radiotherapy, a successful combination therapy in a patient with lung cancer: A case report. Mol. Clin. Oncol. 2019, 11, 50–54.
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193.
- Mayordomo, C.; García-Recio, S.; Ametller, E.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Vinyals, L.; Casas, I.; Gascón, P.; Almendro, V. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J. Cell. Physiol. 2012, 227, 1358–1366.
- Garcia-Recio, S.; Fuster, G.; Fernandez-Nogueira, P.; Pastor-Arroyo, E.M.; Park, S.Y.; Mayordomo, C.; Ametller, E.; Mancino, M.; Gonzalez-Farre, X.; Russnes, H.G.; et al. Substance P Autocrine Signaling Contributes to Persistent HER2 Activation That Drives Malignant Progression and Drug Resistance in Breast Cancer. Cancer Res. 2013, 73, 6424–6434.
- Robinson, P.; Kasembeli, M.; Bharadwaj, U.; Engineer, N.; Eckols, K.T.; Tweardy, D.J. Substance P Receptor Signaling Mediates Doxorubicin-Induced Cardiomyocyte Apoptosis and Triple-Negative Breast Cancer Chemoresistance. BioMed Res. Int. 2016, 2016, 1959270.
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301.
- Werge, T. The tachykinin tale: Molecular recognition in a historical perspective. J. Mol. Recognit. 2007, 20, 145–153.
- Bremer, A.A.; Leeman, S.E.; Boyd, N.D. The common C-terminal sequences of substance P and neurokinin A contact the same region of the NK-1 receptor. FEBS Lett. 2000, 486, 43–48.
- Goldsmith, L.E.; Kwatra, M.M. Tachykinin/Substance P/Neurokinin-1 Receptors. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 360–365.
- UniProt. UniProtKB–P20366 (TKN1_HUMAN). Available online: https://www.uniprot.org/uniprot/P20366 (accessed on 12 May 2021).
- UniProt. UniProtKB–Q9UHF0 (TKNK_HUMAN). Available online: https://www.uniprot.org/uniprot/Q9UHF0 (accessed on 21 May 2021).
- UNIProt. UniProtKB–Q86UU9 (TKN4_HUMAN). Available online: https://www.uniprot.org/uniprot/Q86UU9 (accessed on 20 May 2021).
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95.
- NCBI. TAC1 Tachykinin Precursor 1 . Available online: https://www.ncbi.nlm.nih.gov/gene/6863 (accessed on 20 May 2021).
- The Human Gene Database. TAC3 Gene. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TAC3 (accessed on 20 May 2021).
- NCBI. TAC3 Tachykinin Precursor 3 . Available online: https://www.ncbi.nlm.nih.gov/gene/6866 (accessed on 20 May 2021).
- NCBI. TAC4 Tachykinin Precursor 4 . Available online: https://www.ncbi.nlm.nih.gov/gene/255061 (accessed on 20 May 2021).
- Onaga, T. Tachykinin: Recent developments and novel roles in health and disease. Biomol. Concepts 2014, 5, 225–243.
- Groneberg, D.A.; Harrison, S.; Dinh, Q.T.; Geppetti, P.; Fischer, A. Tachykinins in the respiratory tract. Curr. Drug Targets 2006, 7, 1005–1010.
- Joos, G.F.; Pauwels, R.A. Tachykinin receptor antagonists: Potential in airways diseases. Curr. Opin. Pharmacol. 2001, 1, 235–241.
- Joos, G.F.; Van Schoor, J.; Kips, J.C.; Pauwels, R.A. The effect of inhaled FK224, a tachykinin NK-1 and NK-2 receptor antagonist, on neurokinin A-induced bronchoconstriction in asthmatics. Am. J. Respir. Crit. Care Med. 1996, 153, 1781–1784.
- Lördal, M.; Navalesi, G.; Theodorsson, E.; Maggi, C.A.; Hellström, P.M. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin A in small intestine. Br. J. Pharmacol. 2001, 134, 215–223.
- Simonsen, K.B.; Juhl, K.; Steiniger-Brach, B.; Nielsen, S.M. Novel NK (3) receptor antagonists for the treatment of schizophrenia and other CNS indications. Curr. Opin. Drug Discov. Dev. 2010, 13, 379–388.
- Martinez, A.N.; Philipp, M.T. Substance P and antagonists of the neurokinin-1 receptor in neuroinflammation associated with infectious and neurodegenerative diseases of the central nervous system. J. Neurol. Neuromed. 2016, 1, 29–36.
- Fraser, G.L.; Ramael, S.; Hoveyda, H.R.; Gheyle, L.; Combalbert, J. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J. Clin. Endocrinol. Metab. 2016, 101, 417–426.
- FDA. Emend (Aprepitant) Capsule and Oral Suspension Pediatric Postmarketing Pharmacovigilance Review; FDA: Silver Spring, MD, USA, 2017.
- Hargreaves, R.; Ferreira, J.C.; Hughes, D.; Brands, J.; Hale, J.; Mattson, B.; Mills, S. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann. N. Y. Acad. Sci. 2011, 1222, 40–48.
- FDA. Akynzeo FDA Approval History. Available online: https://www.drugs.com/history/akynzeo.html (accessed on 9 April 2022).
- Center for Drug Evaluation and Research. Varubi Tablets 90mg; Food and Drug Administration: Silver Spring, MD, USA, 2015.
- Center for Drug Evaluation and Research. Approval Package for Varubi Injectable Emulsion for Intravenous Use; Administration, F.A.D., Ed.; Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Goldberg, T.; Fidler, B.; Cardinale, S. Rolapitant (Varubi): A Substance P/Neurokinin-1 Receptor Antagonist for the Prevention of Chemotherapy-Induced Nausea and Vomiting. P T Peer Rev. J. Formul. Manag. 2017, 42, 168–172.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
16 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




