Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Since the identification of substance P in 1931, a number of short, highly conserved, bioactive peptides, called tachykinins, have been isolated and investigated, constituting at present one of the largest families of neuropeptides. In humans, tachykinins are expressed throughout the nervous and immune system, with an important role in the regulation of a wide range of physiological processes that include inflammation, nociception, smooth muscle contractility, epithelial secretion and cell proliferation in the nervous, immune, gastrointestinal, respiratory, urogenital and dermal systems.
- cancer
- tachykinin
- tachykinin receptor
- NK-1R
- targeted treatment
- human
- disease
1. The Challenge of Drug-Resistant Tumors
Cancer remains a major public health problem and a leading cause of death worldwide, accounting for more than 19 million new cases and nearly 10 million deaths in 2020 [1]. At the present time, one of the main challenges in the treatment of cancer patients is overcoming resistance, a clinical situation in which the tumor does not respond to treatment (intrinsic or primary resistance) or, although it initially responded, it ends up relapsing and progressing (secondary or acquired resistance).
Tumor progression is characterized by the sequential appearance of genetically altered cell subpopulations, which, under the selective pressure caused by anti-cancer treatments, promote the accumulation of irreversible changes and the proliferation of cell populations resistant to anticancer treatments [2]. This loss of therapeutic response of cancer cells against anti-cancer drugs, or multidrug resistance, can occur during or after treatment and may lead to the development of cross resistance to either structurally or mechanistically different chemotherapeutics.
The causes of multidrug resistance can include mechanisms as diverse as the elevated metabolism of xenobiotics, enhanced efflux of drugs, increased DNA repair capacity, genomic instability, reduced apoptosis or altered proliferation [3,4], which are, to some extent, related to the different hallmarks of cancer, as defined by Hanahan and Weinberg [5] (Figure 1). Nowadays, intrinsic and acquired multidrug resistance represent the main cause of treatment failure for patients with blood or solid tumors, being responsible for over 90% of deaths in oncologic patients treated with conventional chemotherapy or novel targeted treatments [3].
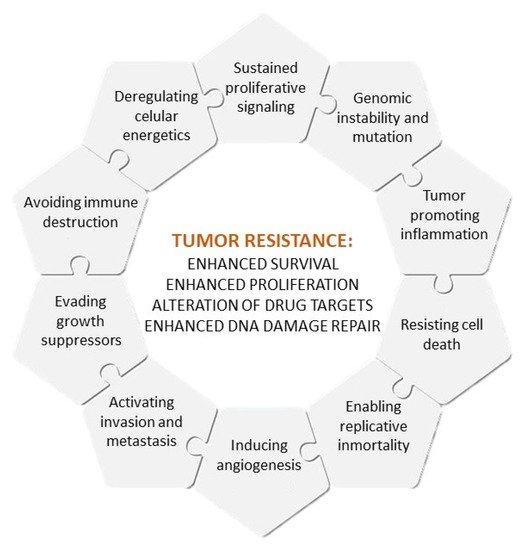
Figure 1. Hallmarks of cancer and causes of multidrug resistance.
Although recent advances in cancer research have allowed for the development of targeted treatments for resistant tumors, most of them have failed in clinical stages, mainly due to their low specificity and high toxicity [6]. For this reason, cancer research is nowadays focused on the development of more effective and safe targeted therapies.
As for other drugs, the development of new therapeutic agents for cancer treatment requires the validation of drug safety and efficacy, which involves multiple phases, from the earliest basic investigation to clinical testing and final authorization. Since this long-term process of authorization of new drugs for cancer treatment, from bench to bedside, is associated with a high risk of failure, timeframe and overall costs [7], the use of known marketed drugs, which have already been assessed for safety and efficacy, for new therapeutic purposes, or drug repurposing, has gained increasing popularity in recent times as a strategy to enhance new pharmaceutical development with rapid clinical translation [8], having proved to shorten the development process by 3–5 years and to increase the success rates from 10% to 25% [7]. So much so that up to 30% of all the current drugs and vaccines approved in recent years by the US Food and Drug Administration (FDA) are repurposed drugs [9], some of which are already being tested to see if they can be useful for the treatment of resistant tumors.
G-protein-coupled receptors (GPCRs) are the largest family proteins targeted by approved drugs, accounting for approximately 17% of FDA agents against human proteins [9,10]. In 2003, aprepitant (EMEND, Merck Sharp & Dohme B.V.) became the first tachykinin-1 receptor (TAC1R, aka neurokinin-1 receptor NK-1R) antagonist approved for the prevention of acute and delayed chemotherapy-induced nausea and vomiting, a side-effect that can affect more than 99% of patients treated with cisplatin [11].
Studies on cancer research also reported that both NK-1R and its preferred ligand (neurokinin 1, NK-1 aka substance P, SP) are overexpressed in a wide variety of malignancies, including leukemia, glioblastoma, astrocytoma, neuroblastoma, melanoma, breast, ovarian, prostate, lung, pancreas and thyroid cancer [12,13], with a role in different driving agents to resistance, such as angiogenesis, cancer cell proliferation, migration and metastasis [12,13,14,15,16]. As a result of a recent clinical report [17] and different in vitro and in vivo studies showing that NK-1R antagonists can exert an antitumor, antiproliferative, anti-survival, antiangiogenic and antimetastatic effect [14,16,18], the inhibition of the NK1-R/SP axis has been proposed as a promising therapeutic approach to battle cancer and cancer resistance [19,20,21], justifying additional investigations that support the reprofiling of marketed NK-1R antagonists, such as aprepitant, as therapeutic agents for cancer treatment, in addition to their use in clinical practice as antiemetic.
2. Tachykinins
Since the identification of substance P in 1931, a number of short, highly conserved, bioactive peptides, called tachykinins, have been isolated and investigated, constituting at present one of the largest families of neuropeptides. In humans, tachykinins are expressed throughout the nervous and immune system, with an important role in the regulation of a wide range of physiological processes that include inflammation, nociception, smooth muscle contractility, epithelial secretion and cell proliferation in the nervous, immune, gastrointestinal, respiratory, urogenital and dermal systems [22].
All members of this family are structurally related peptides characterized by an amidated C-terminal, whose deamidation suppresses peptide activity [23], and a conserved C-terminal sequence -Phe-Xaa-Gly-Leu-Met-NH2, where Xaa represents a hydrophobic amino acid residue [24,25] (Table 1) required for the activation of the corresponding receptor (tachykinin receptor 1/NK-1R, 2/NK-2R or 3/NK-3R) [22], which is responsible for signal transmission from the extracellular environment to the cytoplasm.
Table 1. Main members of the human tachykinin family.
| Gene | Tachykinin | Sequence | Preferred Tachykinin Receptor |
|---|---|---|---|
| TAC1 | Neurokinin 1 (NK1),Substance P (SP) | RPKPQQFFGLM [26] | Neurokinin 1 receptor (NK-1R) |
| Neurokinin A (NKA) Substance K (SK) | HKTDSFVGLM [26] | Neurokinin 2 receptor (NK-2R) | |
| Neuropeptide K (NPK) | DADSSIEKQVALLKALYGHGQISHKRHKTDSFVGLM [26] | Neurokinin 2 receptor (NK-2R) | |
| Neuropeptide γ (NP γ) | MKILVALAVFFLVSTQLFAEEIGANDDLNYWSDWYDSDQIKEELPEPFEHLLQRARRPKPQQFFGLMGKRDADSSIEKQVALLKALYGHGQISHKRHKTDSFVGLMGKRALNSVAYERSAMQNYERRR (1st part)GHGQISHKRHKTDSFVGLM (2nd part) [26] | Neurokinin 2 receptor (NK-2R) | |
| TAC3 | Neurokinin B (NKB)Neuromedin-K | DMHDFFVGLM [27] | Neurokinin 3 receptor (NK-3R) |
| TAC4 | Endokinin A (EKA) | DGGEEQTLSTEAETWVIVALEEGAGPSIQLQLQEVKTGKASQFFGLM [28] | Neurokinin 1 receptor (NK-1R) |
| Endokinin A/B (EKA/B) | GKASQFFGLM [28] | Neurokinin 1 receptor (NK-1R) | |
| Endokinin C (EKC) | KKAYQLEHTFQGLL [28] | Neurokinin 1 receptor (NK-1R) | |
| Endokinin D (EKD) | VGAYQLEHTFQGLL | Neurokinin 1 receptor (NK-1R) |
In humans, tachykinins are encoded by three different genes, TAC1 (tachykinin precursor 1), TAC3 (tachykinin precursor 3) and TAC4 (tachykinin precursor 4) (Table 1):
-
The transcription of the TAC1 gene (NCBI Gene ID: 6863) produces the pre-protachykinin-A (PPTA)-mRNA, which is converted into one of four splice variants coding for a pro-tachykinin polypeptide that contains NK-1 [29], Neurokinin A (NKA, formerly known as substance K) and the NH2-terminally extended forms of NAK neuropeptide K (NPK) and neuropeptide gamma (NPγ) [22,26,30]. These peptides function as neurotransmitters by interacting with nerve receptors and smooth muscle cells [30].
-
TAC3 (NCBI Gene ID: 6866) encodes a preprotein that is further cleaved to generate a mature secreted neuropeptide (neurokinin B, NKB). NKB is primarily expressed in the central and peripheral nervous systems and functions as a neurotransmitter [31]. NKB is a critical central regulator of gonadal function and its alterations are mainly associated with hypogonadotropic hypogonadism [32].
-
Finally, TAC4 (NCBI Gene ID: 255061) produces endokinins (EK) A, A/B, C and D as well as hemokinins [12,28], which are associated with the hematopoietic system and lymphocyte B maturation [12]. TAC4 gene products are thought to regulate different peripheral endocrine and paracrine functions, including blood pressure, the immune system and endocrine gland secretion [33].
3. Tachykinins and Tachykinin Receptors in Human Disease and as Pharmacological Targets
Tachykinins have been associated with different pathological processes, including neurogenic diseases, such as schizophrenia, Alzheimer’s disease and Huntington’s disease [34], as well as acute and chronic inflammation and pain, fibrosis, affective and addictive disorders, functional disorders of the intestine and urinary bladder, infection or cancer [22]. Since tachykinin signaling on targeted cells is mediated by tachykinin receptors, most therapeutic approaches are designed to block ligand–receptor interactions.
3.1. Tachykinins and Tachykinin Receptors in Human Disease
-
Respiratory disorders
Both SP and NKA can be released from airway nerves after noxious stimulation [35], and have a role in respiratory functions, such as the regulation of airway smooth muscle tone, vascular tone and mucus secretion, and immune functions [35]. The overexpression of NK-1R and NK-2R receptors is usually found in inflammatory airway diseases, such as bronchial asthma or chronic obstructive pulmonary disease [35,36], with a role in bronchoconstriction, airway hyperresponsiveness and airway inflammation caused by allergic and nonallergic stimuli, which has prompted the development of both selective and dual-selective NK-1R/NK-2R antagonists that have entered clinic studies with promising results [36,37].
-
Smooth muscle disfunction:
NK-2R is predominantly expressed on smooth muscle, with a role in the contraction of the intestinal, genito-urinary and respiratory tracts. The NK-2R antagonist MEN11420 (nepadutant) has demonstrated, both in preclinical and clinical studies, to be a well-tolerated and a potent, selective and competitive NK-2R inhibitor with an effective and long-lasting anti-spasmogenic effect without affecting basal gastrointestinal motility [38].
-
Central nervous system disorders:
Non-peptide antagonists of NK-3R, which is mainly expressed in the central nervous system, have already shown in preclinical studies their potential utility as a strategy for the treatment of central nervous system disorders [39], such as schizophrenia, major depressive disorder, panic attacks and Parkinson’s disease [39].
Given the role of the SP/NK-1R axis in neuroinflammation associated with different bacterial, viral, parasitic and neurodegenerative diseases of the central nervous system, NK-1R antagonists have recently been proposed as a promising therapeutic agent for the treatment of neuroinflammation [40].
-
Hormonal disorders:
Inactivating mutations in both NKB or its preferred receptor NK-3R have been associated with low gonadotropin-releasing hormone (GnRH) pulse frequency [41]. Both preclinical and clinical studies (phase I clinical trial EUDRACT 2013-004314-17) have shown that the oral administration of the NK-3R antagonist ESN364 is well-tolerated and selectively modulates gonadotropin secretion, which may be of help in the treatment of women’s health disorders with a low risk of menopausal-like adverse events [41].
3.2. Marketed Tachykinin Receptor Antagonists
SP roles in both health and disease have motivated intense research by the pharmaceutical industry to develop selective tachykinin receptor agonists that could be of help in the treatment of human disorders.
Concretely, SP is associated with physiological processes as diverse as hematopoiesis, wound healing, microvasculature permeability, neurogenic inflammation, leukocyte trafficking, cell survival and metastatic dissemination [12], as well as with the regulation of biological processes, such as the dilatation of the arterial vascular system, neuronal survival and degeneration, respiratory function, sensory perception, movement control of gastric motility, salivation, micturition, pain and depression [13].
On March 2006, the FDA approved EMEND® (aprepitant) as the first NK-1R antagonist for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly and moderately (approved on 28 October 2008) emetogenic cancer chemotherapy, as well as for the prevention of post-operative nausea and vomiting (approval on 30 June 2006) in adult patients [42].
Contrary to other previously developed drugs, aprepitant also included a novel nanoparticle formulation to optimize oral absorption that allows for its administration as a water-soluble phosphoryl prodrug form (Ivemend, fosaprepitant) suitable for intravenous administration [43]. Aprepitant selectively blocks the activation of NK-1R in vomiting centers within the central nervous system; thus, since approximately 25% and 50% of patients experience acute or delayed chemotherapy-induced nausea and vomiting, respectively [11], the marketing authorization of this non-peptide NK-1R antagonist represented a great step forward in enhancing the quality of life of oncologic patients who must undergo multiple cycles of chemotherapy [43].
In 2014, the US FDA approved the combination of NK-1R antagonist netupitant plus the 5-HT3R receptor antagonist palonosetron (Akynzeo; Eisai) for the prevention of acute and delayed nausea and vomiting associated with initial and repeated courses of cancer chemotherapy [44].
More recently, in 2015, the FDA also approved aprepitant for patients of 6 months of age and older [42] as well as Varubi© (rolapitant) tablets [45] and rolapitant injectable emulsion [46], as other non-peptide SP/NK-1R antagonists with the same indication as aprepitant/fosaprepitant, but with a better safety profile and lower incidence of adverse events [47].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14092255
This entry is offline, you can click here to edit this entry!
