
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Milan Toma | -- | 2509 | 2022-05-09 16:57:38 | | | |
| 2 | Camila Xu | -36 word(s) | 2473 | 2022-05-10 04:05:51 | | |
Video Upload Options
Computational methods are a cost-effective tool that can be used to evaluate the flow parameters of heart valves. Valve repair and replacement have long-term stability and biocompatibility issues, highlighting the need for a more robust method for resolving valvular disease. For example, while fluid–structure interaction analyses are still scarcely utilized to study aortic valves, computational fluid dynamics is used to assess the effect of different aortic valve morphologies on velocity profiles, flow patterns, helicity, wall shear stress, and oscillatory shear index in the thoracic aorta. It has been analyzed that computational flow dynamic analyses can be integrated with other methods to create a superior, more compatible method of understanding risk and compatibility.
1. Introduction
1.1. Heart Valve Repairs/Devices
1.2. Repair versus Replacement
2. Computational Simulations
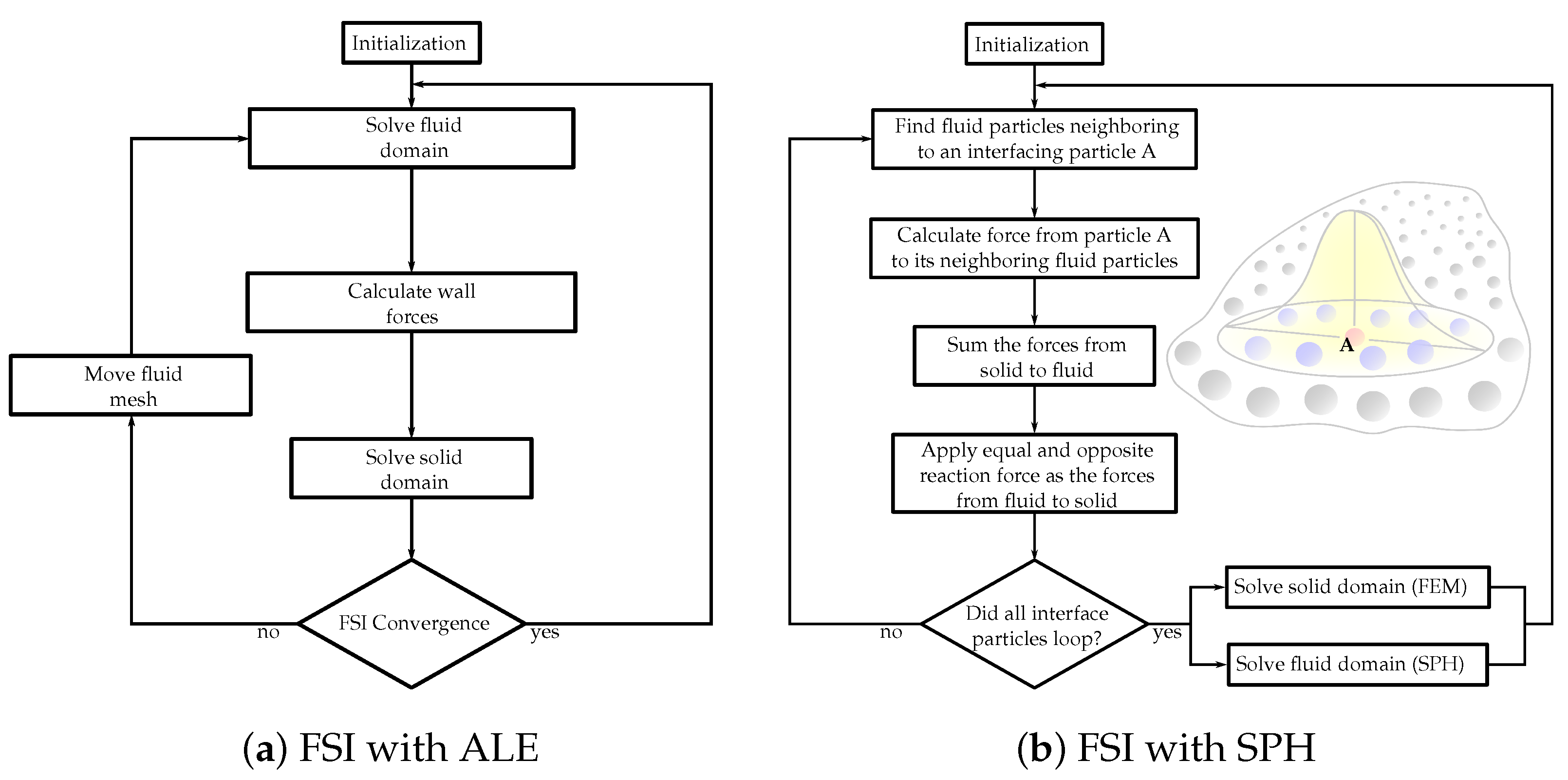 Figure 1. Flowcharts of the FSI solution algorithms with (a) arbitrary Lagrangian–Eulerian (ALE) and (b) smoothed-particle hydrodynamics (SPH) methods.
Figure 1. Flowcharts of the FSI solution algorithms with (a) arbitrary Lagrangian–Eulerian (ALE) and (b) smoothed-particle hydrodynamics (SPH) methods.References
- Fedele, M.; Quarteroni, A. Polygonal surface processing and mesh generation tools for the numerical simulation of the cardiac function. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3435.
- Stephens, S.E.; Kammien, A.J.; Paris, J.C.; Applequist, A.P.; Ingels, N.B.; Jensen, H.K.; Rodgers, D.E.; Cole, C.R.; Wenk, J.F.; Jensen, M.O. In Vitro Mitral Valve Model with Unrestricted Ventricular Access: Using Vacuum to Close the Valve and Enable Static Trans-Mitral Pressure. J. Cardiovasc. Transl. Res. 2022, 1–10.
- Atkins, S.; McNally, A.; Sucosky, P. Mechanobiology in Cardiovascular Disease Management: Potential Strategies and Current Needs. Front. Bioeng. Biotechnol. 2016, 4, 79.
- Dasi, L.P.; Sucosky, P.; De Zelicourt, D.; Sundareswaran, K.; Jimenez, J.; Yoganathan, A.P. Advances in Cardiovascular Fluid Mechanics: Bench to Bedside. Ann. N. Y. Acad. Sci. 2009, 1161, 1–25.
- Toma, M.; Addepalli, D.; Chan-Akeley, R. The Intricacies of Computational Medical Research: An Advanced Study Approach. Recent Dev. Med. Med. Res. 2021, 4, 71–83.
- Wei, Z.A.; Sonntag, S.J.; Toma, M.; Singh-Gryzbon, S.; Sun, W. Computational Fluid Dynamics Assessment Associated with Transcatheter Heart Valve Prostheses: A Position Paper of the ISO Working Group. Cardiovasc. Eng. Technol. 2018, 9, 289–299.
- Food and Drug Administration. Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions, Draft Guidance for Industry and Food and Drug Administration Staff; U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health: Rockville, MD, USA, 2022.
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554.
- Jamari, J.; Ammarullah, M.; Saad, A.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38.
- Toma, M.; Einstein, D.R.; Kohli, K.; Caroll, S.L.; Iv, C.H.B.; Cochran, R.P.; Kunzelman, K.S.; Yoganathan, A.P.; Bloodworth, C. Effect of Edge-to-Edge Mitral Valve Repair on Chordal Strain: Fluid-Structure Interaction Simulations. Biology 2020, 9, 173.
- Jimenez, J.H.; Liou, S.W.; Padala, M.; He, Z.; Sacks, M.; Gorman, R.C.; Gorman, J.H.; Yoganathan, A.P. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J. Thorac. Cardiovasc. Surg. 2007, 134, 1562–1568.
- Sidiki, A.I.; Faybushevich, A.G.; Lishchuk, A.N.; Koltunov, A.N.; Roshchina, E.A. The Carpentier-Edwards Classic And Physio Annuloplasty Rings in Repair of Degenerative Mitral Valve Disease: A Retrospective Study. J. Saudi Heart Assoc. 2020, 32, 224–232.
- Accola, K.D.; Scott, M.L.; Thompson, P.A.; Palmer, G.J.; Sand, M.E.; Ebra, G. Midterm Outcomes Using the Physio Ring in Mitral Valve Reconstruction: Experience in 492 Patients. Ann. Thorac. Surg. 2005, 79, 1276–1283.
- A Tibayan, F.; Rodriguez, F.; Langer, F.; Zasio, M.K.; Bailey, L.; Liang, D.; Daughters, G.T.; Ingels, N.B.; Miller, D. Does septal-lateral annular cinching work for chronic ischemic mitral regurgitation? J. Thorac. Cardiovasc. Surg. 2004, 127, 654–663.
- 2006 WRITING COMMITTEE MEMBERS; Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; De Leon, A.C.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; Lytle, B.W.; Nishimura, R.A.; et al. 2008 Focused Update Incorporated Into the ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease. Circulation 2008, 118, e523–e661.
- Romano, A.M.; Bolling, S.F. Mitral valve repair as an alternative treatment for heart failure patients. Heart Fail. Monit. 2003, 4, 7–12.
- McGee, E.C.; Gillinov, A.M.; Blackstone, E.H.; Rajeswaran, J.; Cohen, G.; Najam, F.; Shiota, T.; Sabik, J.F.; Lytle, B.W.; McCarthy, P.M.; et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2004, 128, 916–924.
- Hung, J.; Papakostas, L.; Tahta, S.A.; Hardy, B.G.; Bollen, B.A.; Duran, C.M.; Levine, R.A. Mechanism of Recurrent Ischemic Mitral Regurgitation After Annuloplasty. Circulation 2004, 110, II-85–II-90.
- Matsunaga, A.; A Tahta, S.; Duran, C.M. Failure of reduction annuloplasty for functional ischemic mitral regurgitation. J. Heart Valve Dis. 2004, 13, 390–398.
- Gelsomino, S.; Lorusso, R.; De Cicco, G.; Capecchi, I.; Rostagno, C.; Caciolli, S.; Romagnoli, S.; Da Broi, U.; Stefàno, P.; Gensini, G.F. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur. Heart J. 2007, 29, 231–240.
- A Tahta, S.; Oury, J.H.; Maxwell, J.M.; Hiro, S.P.; Duran, C.M.G. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J. Heart Valve Dis. 2002, 11, 11–18.
- Yousefnia, M.A.; Mandegar, M.H.; Roshanali, F.; Alaeddini, F.; Amouzadeh, F. Papillary Muscle Repositioning in Mitral Valve Replacement in Patients With Left Ventricular Dysfunction. Ann. Thorac. Surg. 2007, 83, 958–963.
- Minardi, G.; Manzara, C.; Pulignano, G.; Luzi, G.; Maselli, D.; Casali, G.; Musumeci, F. Rest and Dobutamine stress echocardiography in the evaluation of mid-term results of mitral valve repair in Barlow’s disease. Cardiovasc. Ultrasound 2007, 5, 17.
- Seeburger, J.; Borger, M.; Doll, N.; Walther, T.; Passage, J.; Falk, V.; Mohr, F.W. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur. J. Cardio-Thorac. Surg. 2009, 36, 532–538.
- Minami, K.; Kado, H.; Sai, S.; Tatewaki, H.; Shiokawa, Y.; Nakashima, A.; Fukae, K.; Hirose, H. Midterm results of mitral valve repair with artificial chordae in children. J. Thorac. Cardiovasc. Surg. 2005, 129, 336–342.
- Croft, L.R.; Jimenez, J.H.; Gorman, R.C.; Gorman, J.H.; Yoganathan, A.P. Efficacy of the Edge-to-Edge Repair in the Setting of a Dilated Ventricle: An In Vitro Study. Ann. Thorac. Surg. 2007, 84, 1578–1584.
- Espino, D.M.; Hukins, D.W.L.; Shepherd, D.E.T.; Buchan, K.G. Mitral valve repair: An in-vitro comparison of the effect of surgical repair on the pressure required to cause mitral valve regurgitation. J. Heart Valve Dis. 2006, 15, 375–381.
- Melillo, F.; Baldetti, L.; Beneduce, A.; Agricola, E.; Margonato, A.; Godino, C. Mitral valve surgery after a failed MitraClip procedure. Interact. Cardiovasc. Thorac. Surg. 2020, 32, 380–385.
- Askov, J.B.; Honge, J.L.; Jensen, M.O.; Nygaard, H.; Hasenkam, J.M.; Nielsen, S.L. Significance of force transfer in mitral valve–left ventricular interaction: In vivo assessment. J. Thorac. Cardiovasc. Surg. 2013, 145, 1635–1641.e1.
- Toma, M.; Jensen, M.; Einstein, D.R.; Yoganathan, A.P.; Cochran, R.P.; Kunzelman, K.S. Fluid–Structure Interaction Analysis of Papillary Muscle Forces Using a Comprehensive Mitral Valve Model with 3D Chordal Structure. Ann. Biomed. Eng. 2015, 44, 942–953.
- Dalrymple-Hay, M.J.; Bryant, M.; A Jones, R.; Langley, S.M.; A Livesey, S.; Monro, J.L. Degenerative mitral regurgitation: When should we operate? Ann. Thorac. Surg. 1998, 66, 1579–1583.
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394.
- Flameng, W.; Herijgers, P.; Bogaerts, K. Recurrence of Mitral Valve Regurgitation After Mitral Valve Repair in Degenerative Valve Disease. Circulation 2003, 107, 1609–1613.
- Asakai, H.; Kaneko, Y.; Kaneko, M.; Misaki, Y.; Achiwa, I.; Hirata, Y.; Kato, H. Acute Progressive Mitral Regurgitation Resulting From Chordal Rupture in Infants. Pediatr. Cardiol. 2011, 32, 634–638.
- Maslow, A.D.; Poppas, A.; Apostolidou, E. Primary mitral valve regurgitation: Update and review. Glob. Cardiol. Sci. Pract. 2017, 2017, e201703.
- Northrup, W.F. Mitral valve repair: We must do a better job. Curr. Cardiol. Rep. 2005, 7, 94–100.
- Suri, R.M.; Schaff, H.V.; Dearani, J.A.; Sundt, T.M.; Daly, R.C.; Mullany, C.J.; Enriquez-Sarano, M.; Orszulak, T.A. Recurrent mitral regurgitation after repair: Should the mitral valve be re-repaired? J. Thorac. Cardiovasc. Surg. 2006, 132, 1390–1397.
- Aphram, G.; De Kerchove, L.; Mastrobuoni, S.; Navarra, E.; Solari, S.; Tamer, S.; Baert, J.; Poncelet, A.; Rubay, J.; Astarci, P.; et al. Re-repair of the failed mitral valve: Insights into aetiology and surgical management. Eur. J. Cardio-Thorac. Surg. 2018, 54, 774–780.
- Lio, A.; Miceli, A.; Varone, E.; Canarutto, D.; Di Stefano, G.; Della Pina, F.; Gilmanov, D.; Murzi, M.; Solinas, M.; Glauber, M. Mitral valve repair versus replacement in patients with ischaemic mitral regurgitation and depressed ejection fraction: Risk factors for early and mid-term mortality. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 64–69.
- Takagi, H.; Umemoto, T. Similar Survival After Repair vs Replacement for Ischemic Mitral Regurgitation. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 748–756.
- A Moore, R.; Gillinov, A.M.; Burns, D.J.; Pettersson, G.B.; Wierup, P. Techniques for Mitral Valve Re-repair. Oper. Tech. Thorac. Cardiovasc. Surg. 2021, 26, 42–65.
- Veerappan, M.; Cheekoty, P.; Sazzad, F.; Kofidis, T. Mitral valve re-repair vs replacement following failed initial repair: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2020, 15, 304.
- El Oumeiri, B.; Boodhwani, M.; Glineur, D.; De Kerchove, L.; Poncelet, A.; Astarci, P.; Pasquet, A.; Vanoverschelde, J.-L.; Verhelst, R.; Rubay, J.; et al. Extending the Scope of Mitral Valve Repair in Rheumatic Disease. Ann. Thorac. Surg. 2009, 87, 1735–1740.
- Sharma, A.; Agrawal, S.; Goel, S.; Borer, J.S. Surgical Treatment of Ischemic Mitral Regurgitation: Valve Repair Versus Replacement. Curr. Cardiol. Rep. 2017, 19, 3.
- Soares, J.S.; Feaver, K.R.; Zhang, W.; Kamensky, D.; Aggarwal, A.; Sacks, M.S. Biomechanical Behavior of Bioprosthetic Heart Valve Heterograft Tissues: Characterization, Simulation, and Performance. Cardiovasc. Eng. Technol. 2016, 7, 309–351.
- Ge, L.; Jones, S.C.; Sotiropoulos, F.; Healy, T.M.; Yoganathan, A.P. Numerical Simulation of Flow in Mechanical Heart Valves: Grid Resolution and the Assumption of Flow Symmetry. J. Biomech. Eng. 2003, 125, 709–718.
- Toma, M.; Oshima, M.; Takagi, S. Decomposition and parallelization of strongly coupled fluid-structure interaction linear subsystems based on the Q1/P0 discretization. J. Comput. Struct. 2016, 173, 84–94.
- Gao, H.; Feng, L.; Qi, N.; Berry, C.; Griffith, B.; Luo, X. A coupled mitral valve—left ventricle model with fluid–structure interaction. Med. Eng. Phys. 2017, 47, 128–136.
- Feng, L.; Gao, H.; Griffith, B.; Niederer, S.A.; Luo, X. Analysis of a coupled fluid-structure interaction model of the left atrium and mitral valve. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3254.
- Peskin, C.S. Numerical analysis of blood flow in the heart. J. Comput. Phys. 1977, 25, 220–252.
- Tang, D.; Yang, C.; Kobayashi, S.; Zheng, J.; Vito, R.P. Effect of Stenosis Asymmetry on Blood Flow and Artery Compression: A Three-Dimensional Fluid-Structure Interaction Model. Ann. Biomed. Eng. 2003, 31, 1182–1193.
- Wong, K.; Fong, F.; Wang, D. Computational evaluation of smoothed particle hydrodynamics for implementing blood flow modelling through CT reconstructed arteries. J. Ray Sci. Technol. 2017, 25, 213–232.
- Nasar, A. Eulerian and Lagrangian Smoothed Particle Hydrodynamics as Models for the Interaction of Fluids and Flexible Structures in Biomedical Flows. Ph.D. Thesis, The University of Manchester (United Kingdom), PQDT-UK & Ireland, Manchester, UK, 2016.
- Hron, J.; Mádlík, M. Fluid-structure interaction with applications in biomechanics. Nonlinear Anal. Real World Appl. 2007, 8, 1431–1458.
- Al-Saad, M.; Suarez, C.A.; Obeidat, A.; Bordas, S.P.A.; Kulasegaram, S. Application of Smooth Particle Hydrodynamics Method for Modelling Blood Flow with Thrombus Formation. Comput. Model. Eng. Sci. 2020, 122, 831–862.
- Le, T.B.; Usta, M.; Aidun, C.; Yoganathan, A.; Sotiropoulos, F. Computational Methods for Fluid-Structure Interaction Simulation of Heart Valves in Patient-Specific Left Heart Anatomies. Fluids 2022, 7, 94.




