Computational methods are a cost-effective tool that can be used to evaluate the flow parameters of heart valves. Valve repair and replacement have long-term stability and biocompatibility issues, highlighting the need for a more robust method for resolving valvular disease. For example, while fluid–structure interaction analyses are still scarcely utilized to study aortic valves, computational fluid dynamics is used to assess the effect of different aortic valve morphologies on velocity profiles, flow patterns, helicity, wall shear stress, and oscillatory shear index in the thoracic aorta. It has been analyzed that computational flow dynamic analyses can be integrated with other methods to create a superior, more compatible method of understanding risk and compatibility.
- heart valves
- mitral valve
- tricuspid valve
- aortic valve
1. Introduction
1.1. Heart Valve Repairs/Devices
Numerous studies have been executed to investigate the impact of medical devices on decreasing the stress in diverse MV regions [10][11][12][46,47,48]. While the physio ring is regarded as an improved rendition of the traditional rigid ring, and the physio ring is more widely employed, long-term results of repair for degenerative MV disease with the classic and physio rings are equivalent [12][48]. However, the low incidence of reoperation and late cardiac events indicates that the physio ring, with its intrinsic flexibility, presents an indisputable benefit in the application of remodeling strategies in MV reconstruction [13][49]. Some therapies of choice for chronic ischemic mitral regurgitation annul active annular movement and immobilize the posterior leaflet. In a model of chronic ischemic mitral regurgitation, septal–lateral annular cinching sought to uphold regular annular and leaflet dynamics was tested [14][50]. A decrease of the annulus with an undersized ring has once seemed to be the select surgical choice to rectify ischemic mitral incompetence [15][16][51,52]. Nonetheless, numerous investigations uncovered substantial residual and repeat rates of mild to severe mitral incompetence in 30% of patients within 6 months of surgery [17][18][19][20][21][53,54,55,56,57]. Mitral valve replacement strategies in patients with left ventricular dysfunction are often chaperoned with other techniques for more promising left ventricular remodeling compared with total retention of the mitral subvalvular apparatus during MV replacement [22][58]. Surgical repair is the most routine procedure used to rectify mitral regurgitation. However, the effectiveness of other techniques is still examined. The efficacy of a procedure is specified using an immense combination of factors, such as the durability of the repaired valve as well as the valve’s function and hemodynamics under stress states. Thus, a myriad of studies are carried out to assess these parameters at follow-ups [23][24][25][26][59,60,61,62]. By approximating edge-to-edge repair (to repair ruptured/elongated chords) with chordal replacement, it was discovered that edge-to-edge repair and chordal replacement are sufficiently suited for the restoration of both the anterior and posterior leaflets [27][63]. Regardless, among patients experiencing transcatheter MV edge-to-edge repair with the MitraClip device, a pertinent ratio (2–6%) requires open MV surgery within 1 year after unsuccessful clip implantation [28][64]. Both in vivo and in silico examinations evaluated the combined force transfer from the papillary muscle tips to the MV via the chordae tendineae, and thereby quantified the force shared through the papillary–chordal complex to augment left ventricular ejection [29][30][65,66].1.2. Repair versus Replacement
The outcomes underscore the significance of early detection and resviearchw of mitral regurgitation [31][67]. The most satisfactory short-term and long-term results are gained in asymptomatic patients worked on in state-of-the-art repair centers with low operative mortality and high repair rates [32][68]. The durability of a successful mitral reconstruction for degenerative MV condition is not consistent, and this should be accepted when asymptomatic patients are proposed early MV repair [33][69]. Early diagnosis and surgery are paramount as a life-saving standard for infants with acute MV chordal rupture [25][34][61,70]. The unique vision of staging of the valvular diseases, newer predictors, and controversy of “watchful waiting” versus “early surgical intervention” for severe, asymptomatic, primary mitral regurgitation are examined in a study that outlines the current interpretation of primary, degenerative mitral regurgitation concerning etiology, complete examination, natural history, and control [35][71]. Based upon a sounder knowledge of the natural history of mitral regurgitation, the unsatisfactory effects of medical therapies, the adverse consequence of anomalous left ventricular dimensions and function, and manifestations of long-term survival, a directive presently exists for early surgical repair of mitral regurgitation before the start of symptoms and considerable left ventricular dysfunction [36][72]. New valve pathology after a repair oftentimes results in recurrent mitral regurgitation. Successive mitral re-repair is conducted in nearly half of patients and is associated with outstanding survival, enhanced ejection fraction, and more significant regression in ventricular proportions compared with valve replacement [37][38][73,74]. However, an observational analysis found that MV repair in coronary artery bypass grafting patients with ischaemic mitral regurgitation and depressed left ventricular ejection fraction is not incomparable to mitral valve replacement concerning operative early mortality and mid-term survival [39][75]. A meta-analysis of randomized controlled trials and adjusted observational studies demonstrated that for patients with ischemic mitral regurgitation, MV repair seems to be unassociated with a noteworthy reduction in both early and late all-cause mortality compared with MV replacement [40][76]. The mechanisms of MV repair failure as well as aspects that meaningfully impact the probability of a successful re-repair can be located in [41][77]. When comparing MV re-repair versus replacement following failed initial repair, it was uncovered that they are associated with comparable postoperative outcomes [42][78]. Repair of rheumatic MVs has been met with narrow success. Due to residual diseased leaflet tissue, the hemodynamic obstruction continually endures after repair. An assertive strategy to rheumatic MV repair with extreme excision of the diseased leaflets area, and subvalvular apparatus and subsequent reconstruction, intending to extract all diseased valvular tissue, was devised and executed [43][79]. Data comparing processes of MV repair and replacement for ischemic mitral regurgitation are primarily restricted to small, non-randomized retrospective trials [44][80]. The only randomized trial data to investigate this topic indicated no distinction in mortality with either replacement or repair; however, the replacement was shown to be invariably associated with higher rates of mitral regurgitation recurrence [44][80]. Regardless, the use of replacement heart valves persists to grow due to the raised preponderance of valvular heart disorders resulting from an aging population [45][81].2. Computational Simulations
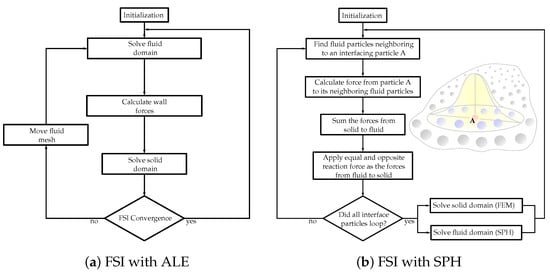
To determine the coupling between the fluid and structural domains, FSI strategies are utilized. Particularly in computational simulations meant to imitate the functions inside the human body, intricate dynamics are present, e.g., heart valves opening and closing every second interacting with blood flow. Consequently, for physiologically authentic simulations, the fluid dynamics associated with the valves, the structural mechanics of the valves, and tissue characteristics, should be modeled concurrently. Nonetheless, standard FSI studies present several challenges, e.g., considerable extra computation time. FSI simulations can be separated into three significant classifications: (1) Pseudo-state simulations are generally used to investigate the downstream flow domains of heart valves under the supposition that the valve is unmoving, and they can be modeled utilizing ordinary computational fluid dynamics strategies for flow fields [46][109]. (2) One-way FSI lets heart valves move under a stipulated geometric deformation. The prescribed structure dynamic movement impacts the fluid flow but not contrariwise. In two-way FSI (3), the most demanding type of FSI simulation, the structural and fluid fields influence one another. The structural model of a two-way FSI solver requires adequately representing material properties and the interaction between the leaflets and the surrounding fluid. Naturally, most two-way FSI solvers can solve one-way FSI problems. Two techniques are utilized for the coupling between the fluid and structure domains. (A) Partitioned technique: The fluid and solid domains are treated individually with two separate solvers (Figure 1a). Communication between the two solvers is passed along their domain interface. Since each domain is solved employing a different solver, autonomous numerical algorithms can be involved to solve the fluid and solid equations. Consequently, less memory storage is demanded compared to the monolithic approach. However, in the FSI heart valves simulations, which typically include large deformations, this technique tends to face converge issues due to stability problems [46][109]. (B) Monolithic approach: The fluid and structural domains are solved simultaneously by discretizing the problem into a single system of equations employing a single numerical algorithm [47][110]. This generates fewer convergence issues since the joint impact of the two domains on one another is incorporated directly. However, for extensive 3D problems, with a high number of degrees of freedom, a prohibitive quantity of memory storage is required.