Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luís R. Silva | -- | 2816 | 2022-04-29 10:16:16 | | | |
| 2 | Camila Xu | -1 word(s) | 2815 | 2022-04-29 10:59:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, L.; Gonçalves, A.C.; , .; Flores-Félix, J.; Falcão, A.; Alves, G. Phenolic Compounds and Its Linkage. Encyclopedia. Available online: https://encyclopedia.pub/entry/22491 (accessed on 07 February 2026).
Silva L, Gonçalves AC, , Flores-Félix J, Falcão A, Alves G. Phenolic Compounds and Its Linkage. Encyclopedia. Available at: https://encyclopedia.pub/entry/22491. Accessed February 07, 2026.
Silva, Luís, Ana Carolina Gonçalves, , J.d. Flores-Félix, Amílcar Falcão, Gilberto Alves. "Phenolic Compounds and Its Linkage" Encyclopedia, https://encyclopedia.pub/entry/22491 (accessed February 07, 2026).
Silva, L., Gonçalves, A.C., , ., Flores-Félix, J., Falcão, A., & Alves, G. (2022, April 29). Phenolic Compounds and Its Linkage. In Encyclopedia. https://encyclopedia.pub/entry/22491
Silva, Luís, et al. "Phenolic Compounds and Its Linkage." Encyclopedia. Web. 29 April, 2022.
Copy Citation
Medicinal plants, along with cereals, fruits, herbs, and vegetables, are the main sources of phenolic compounds, of which nearly 10,000 have been reported in nature to date. They are secondary metabolites produced by plants to protect them from abiotic factors (e.g., drought, extreme temperatures, floods, heavy metals, pH, radiation, salinity, and soils) and biotic factors, such as animals and pathogens attack.
phenolic compounds
exercise
recovery
1. Phenolic Compounds
Medicinal plants, along with cereals, fruits, herbs, and vegetables, are the main sources of phenolic compounds, of which nearly 10,000 have been reported in nature to date [1][2]. They are secondary metabolites produced by plants to protect them from abiotic factors (e.g., drought, extreme temperatures, floods, heavy metals, pH, radiation, salinity, and soils) and biotic factors, such as animals and pathogens attack [3]. In addition, they are considered to be mainly responsible for the organoleptic characteristics (e.g., aroma, flavour, and colour) of plants [4]. To facilitate their identification, they can be classified into five different subgroups based on their structure (at least one phenol ring), namely, (i) phenolic acids (which are further subdivided into hydroxybenzoic and hydroxycinnamic acids), (ii) flavonoids (subdivided into flavonols, flavan-3-ols, flavones, flavanones, isoflavones, flavanonols, and anthocyanidins), (iii) coumarins, (iv) lignans and (v) stilbenes (Figure 1) [5]. Among them, flavonoids are the most abundant in plants [6]. Flavonols are mostly found in apples, berries, grapes, broccoli, lettuce, onions, spinach, and tomatoes, while flavan-3-ols are mainly found in apples, bananas, blueberries, kiwi, peaches, pears, and their juices and jams, cereals, coca, broad beans, lentils, red wine and tea [7][8][9][10][11]. On the other hand, celery, tea, parsley, red pepper, and oranges contain significant amounts of flavones, while lemons and bitter oranges are rich in flavanones [8][9][10]. Isoflavones are mainly found in soy and soy products, lentils, beans and peas, and flavanonols, in lemons, grapes, oranges, and mint [7][8][9][11]. Finally, anthocyanins are largely identified in berry fruits and their juices and jams, pigmented cereals, purple corn, red cabbage, and red wine [7][11]. Regarding other phenolics, hydroxybenzoic acids have been reported in berries, onions, and black radish, whereas hydroxycinnamic acids are abundant in apples, berries, pears, plums, artichokes, carrots, chicory, eggplant, lettuce, wheat, and coffee [4][7][8][9]. Bison, cinnamon, lavender, sweet woodruff, and strawberries contain coumarins, while lignans are mostly found in sesame and flaxseeds [7][11][12]. Finally, stilbenes are predominant in blueberries, grapes, peanuts, and sorghum [13].
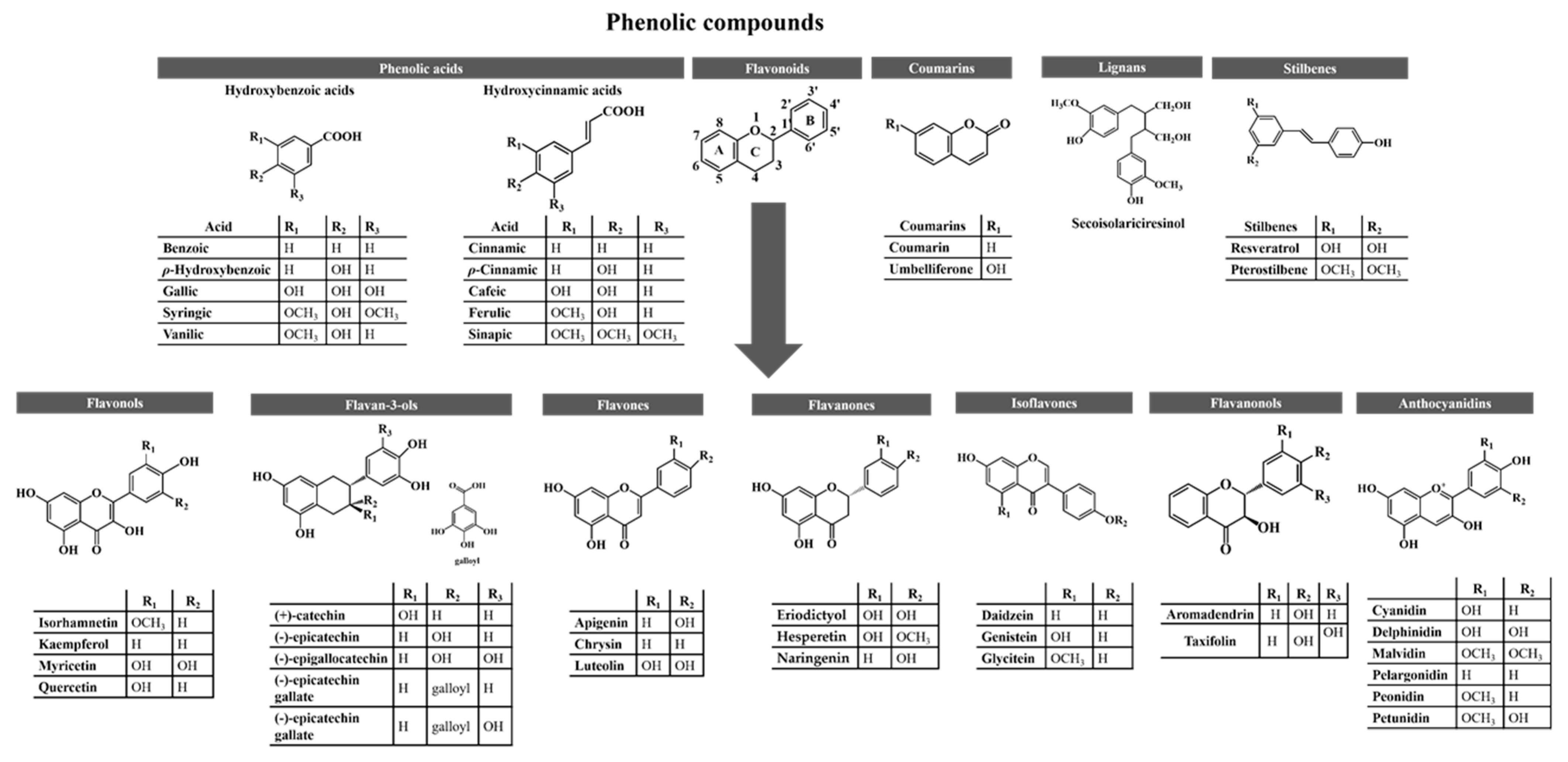 Figure 1. Main phenolic compounds. Non-flavonoids comprise coumarins, phenolic acids, lignans and stilbenes, while flavonoids include flavonols, flavan-3-ols, flavones, isoflavones, flavanonols and anthocyanidins. Among them, flavonoids are the most found in nature.
Figure 1. Main phenolic compounds. Non-flavonoids comprise coumarins, phenolic acids, lignans and stilbenes, while flavonoids include flavonols, flavan-3-ols, flavones, isoflavones, flavanonols and anthocyanidins. Among them, flavonoids are the most found in nature.As mentioned earlier, their structure (Figure 1) allows them to readily neutralize free radicals and reactive species, interfere with signaling pathways, and reduce pro-inflammatory markers [3][14][15]. Therefore, it is not surprising that their scientific interest, consumption, and use in dietary supplements, functional foods, and pharmaceuticals are increasing worldwide [16]. However, although understanding the stability, solubility, bioavailability, and bioactivity of phenolics is not an easy task, it is essential because their health benefits depend mainly on the amount ingested and bioavailability [17]. In general, the bioavailability of different phenolics varies widely, and the most abundant phenolics in the diet are not necessarily the most active in vivo, either owing to their poor absorption by the intestine, rapid excretion, high metabolism, and/or lower intrinsic activity [9]. Nowadays, it is estimated that the absorption rates of the most consumed phenolics range from 2.7–12.2% for lignans, 8–72% for hydroxycinnamic acids, 33–100% for isoflavones, 12–41% for flavonols and flavones, 11–16% for flavanones, 2–8% for flavan-3-ols and 2.4–55% for anthocyanins [11][18][19][20][21][22]. Nevertheless, some studies reported that these percentages may eventually be higher [21][23]. In an attempt to improve the bioavailability and bioactivity of phenolic compounds, various efforts have been made and discussed. Among the alternatives, their encapsulation with carbohydrates (e.g., cellulose derivatives and maltodextrins), lipids (e.g., emulsifiers and waxes), natural gums (e.g., alginates and gum arabic), and/or with proteins (e.g., dairy proteins, gelatin, and soy proteins) seem to be the most promising strategies [17][24].
In addition, it is important to consider that bioavailability rates largely depend on the samples maturity stage, genotype, and agronomic conditions, which in turn, directly influence phenolic content, food processing, pH, solubility, chemical structure, and sugar moieties [22][25]. Furthermore, it is also important to consider interactions with other components present in food matrix, and the inter-individual variability between individuals (e.g., gender, age, food intolerances, genetic profile, enzymatic activity, pathological and/or physiological state of intestinal flora), lifestyle, and dietary habits [10][25].
Bioavailability of phenolics depends on different processes, including their release from the food matrix, gastrointestinal absorption, and presystemic metabolism; among them, intestinal absorption is considered the rate-limiting step and is the main culprit in reducing the bioavailability [6][26]. It begins in the mouth (Figure 2) with chewing, where the action of the oral microbiota initiates the metabolism of glycosylated compounds [20]. In the stomach, only a few compounds are hydrolyzed and deconjugated, with most remaining intact [27]. Here, some aglycones and simpler phenolic acids can be absorbed (bioaccessibility) [26]. The other pass to the small intestine, where cleavage and release of aglycones occur by the action of various digestive enzymes, with the activity of cytosolic β-glucosidase and lactase-phlorizin hydrolase being prominent [9]. Then, phenolics can be taken up by passive diffusion or transporters [27]. Some properties, such as lipophilicity, molecular weight, and glycosylation pattern, may affect their transport and permeability [6]. For example, isoflavones and gallic acid are readily absorbed due to their low molecular weight; on the other hand, proanthocyanins must first be degraded due to their high molecular weight [28][29]. The high lipophilicity of aglycones allows them to easily pass through epithelial cells by passive diffusion, whereas polymers, glycosides, and esters cannot pass through the membrane by passive diffusion and require transporters [28]. Quercetin glycosides, for example, require the action of sodium-dependent glucose cotransporter-1, while anthocyanins require glucose transporters 1 and 3 [6][30].
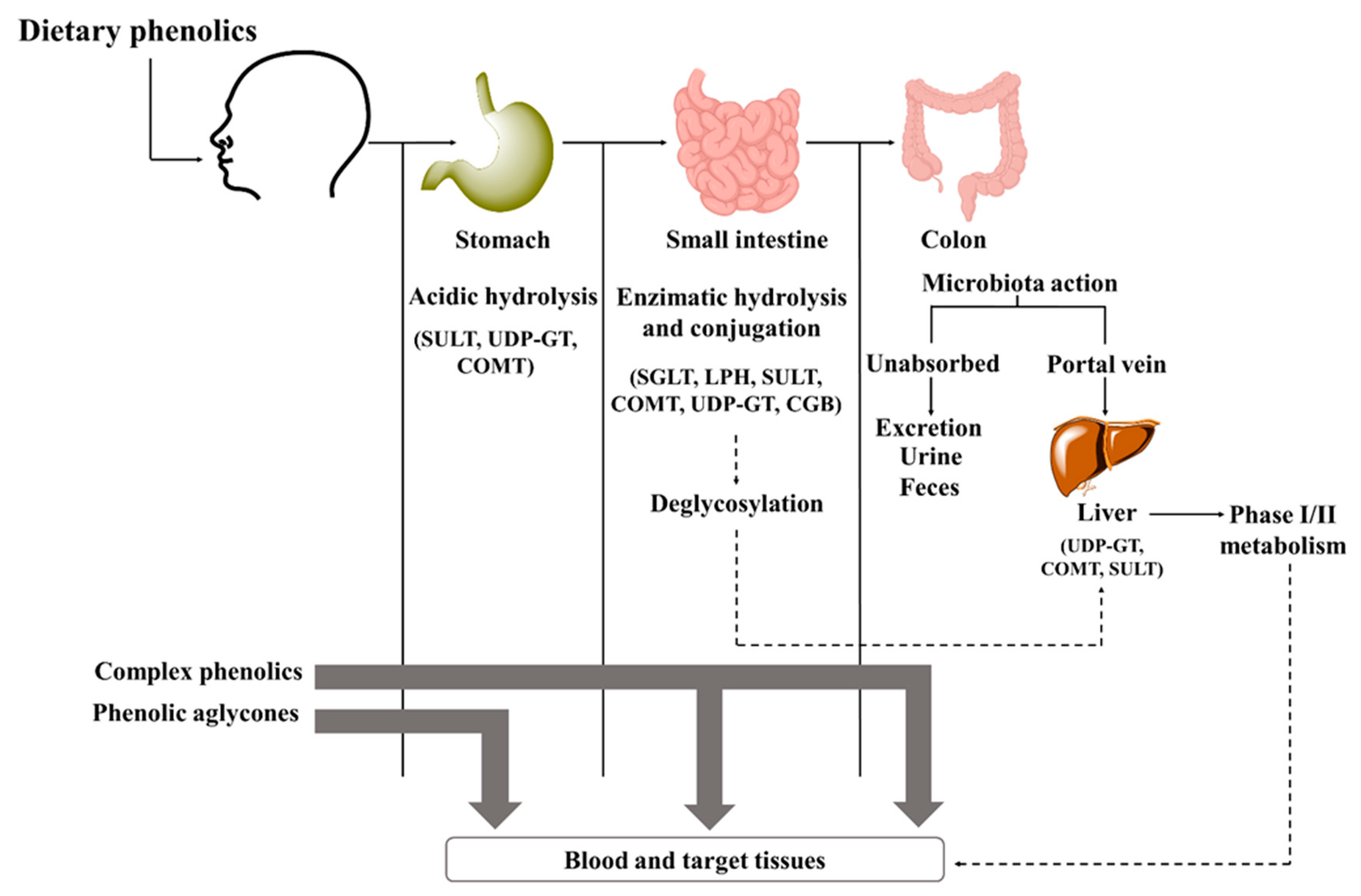 Figure 2. Simplified steps of the metabolic pathways involved in the bioavailability of phenolics in the human body after consumption. CGB—cytosolic β-glucosidase; SULT—sulfotransferase; UDP-GT—glucuronosyltransferase; COMT—catechol-O-methyl transferase; SGLT—sodium-dependent glucose cotransporters; LPH—lactase-phlorizin hydrolase.
Figure 2. Simplified steps of the metabolic pathways involved in the bioavailability of phenolics in the human body after consumption. CGB—cytosolic β-glucosidase; SULT—sulfotransferase; UDP-GT—glucuronosyltransferase; COMT—catechol-O-methyl transferase; SGLT—sodium-dependent glucose cotransporters; LPH—lactase-phlorizin hydrolase.Nevertheless, the greatest absorption rate is observed in the colon [31]. Here, the gut microbiota catalyzes a series of reactions, including decarboxylation, demethylation, dihydroxylation, and hydrolysis, and transforms unabsorbed phenolics into bioavailable metabolites [27]. Usually, flavones and flavanones are degraded to hydroxy-phenylpropionic acids and polymeric phenolic compounds, while anthocyanins and proanthocyanidins are metabolizated to low molecular weight phenolic acids, flavonols converted into hydroxyphenylacetic acids, and flavanols into phenylvalerolactones and hydroxyphenylpropionic acids [24][32]. These acids are further metabolized to benzoic acid derivatives [32][33]. In the liver, they undergo some degree of phase I and/or phase II biotransformation reactions. Phase I includes hydrolysis, oxidation, and reduction reactions carried out by cytochrome P450 enzymes, while phase II aims to increase hydrophilicity before excretion [27]. Finally, metabolites may return to the small intestine via enterohepatic recirculation, be further absorbed by enterocytes, and return to the liver, or be eliminated [20].
2. The Linkage between Phenolics Structure and Health Benefits
It is clear from the literature, that phenolics have several beneficial effects on human health, including remarkable antioxidant, vasoactive and anti-inflammatory activities, and the ability to interact with enzymes, and cell receptors [34].
2.1. Antioxidant Effects of Phenolics
Regarding antioxidant mechanisms, different arrangements of functional groups in the core nuclear structure can confer metal chelation, radical scavenging, and/or oxidative activity to phenolics [35]. Although the role of free radicals and RNOS in human metabolism is undeniable, namely in the regulation of gene expression and cell growth, immune responses, and signal transduction pathways, their overproduction and consequent accumulation lead to DNA, lipids and proteins damage, necrosis, and useless inflammatory responses and promote the onset of several diseases, such as cancer, cardiovascular and neurological pathologies [14]. Unlike synthetic antioxidants, whose belief is that their constant intake is harmful and has undesirable effects, phenolics have already shown a notable ability to reduce oxidative stress levels with little or no negative effects [36].
Phenolics are effective in controlling oxidative stress and restoring the redox homeostasis due to their ability to (i) neutralize and/or reduce free radicals and the formation of reactive species, (ii) chelate trace elements involved in the formation of these pro-oxidant species, (iii) modulate the activity of related enzymes in cell signaling cascades, and (iv) stimulate the endogenous defense system by stimulating the action of intracellular antioxidant enzymes (e.g., catalase, glutathione, and superoxide dismutase) [5][37]. In this way, phenolics can decrease the production of pro-apoptotic multidomain effectors Bax and Bak protein, promoting an increase in the ratio of Bcl2–Bcl-xL/Bax–Bak, as well as stimulate the nuclear factor erythroid 2-related factor 2, and thereby, regulate the transcription of various antioxidant genes [38].
These effects are primarily attributed to their aromatic rings and the presence of several hydroxyl groups, which make them good hydrogen or electron donors [25][39]. However, the spatial position and the number of hydroxyl groups can affect their antioxidant potential [40]. As far as know, phenolics with aromatic rings with two hydroxyl groups in the ortho-position (catechol group) are better antioxidants than those who only have aromatic rings with a single hydroxyl group (simple phenol groups) [5]. Procyanidin dimers followed by flavan-3-ols and flavanols are known to be the best antioxidants, followed by hydroxycinnamic acids and finally by simple phenolic acids [37].
As for phenolic acids, due to the CH=CH-COOH group and the 7,8-double bond in hydroxycinnamic acids, they are more efficient in reducing radicals and reactive species than hydroxybenzoic acids, which have only one COOH group (Figure 3A) [37]. In addition, the substitution of the carboxyl group with O-alkyl ester groups can also increase the antioxidant potential of phenolic acids, as well as the substitution with hydroxyl groups on the benzoic ring at the ortho and/or para-positions [41][42]. In contrast to ortho-methoxy groups, ortho-hydroxyl groups enhance these benefits [40]. On the other hand, the electron-withdrawing activity of the carboxyl group in phenolic acids reduces their capturing abilities [42]. Among phenolics, gallic and rosmarinic acids are considered to possess the highest antioxidant power (Trolox equivalent antioxidant capacity (TEAC) values of 3.62 and 4.5 mM, respectively) [37].
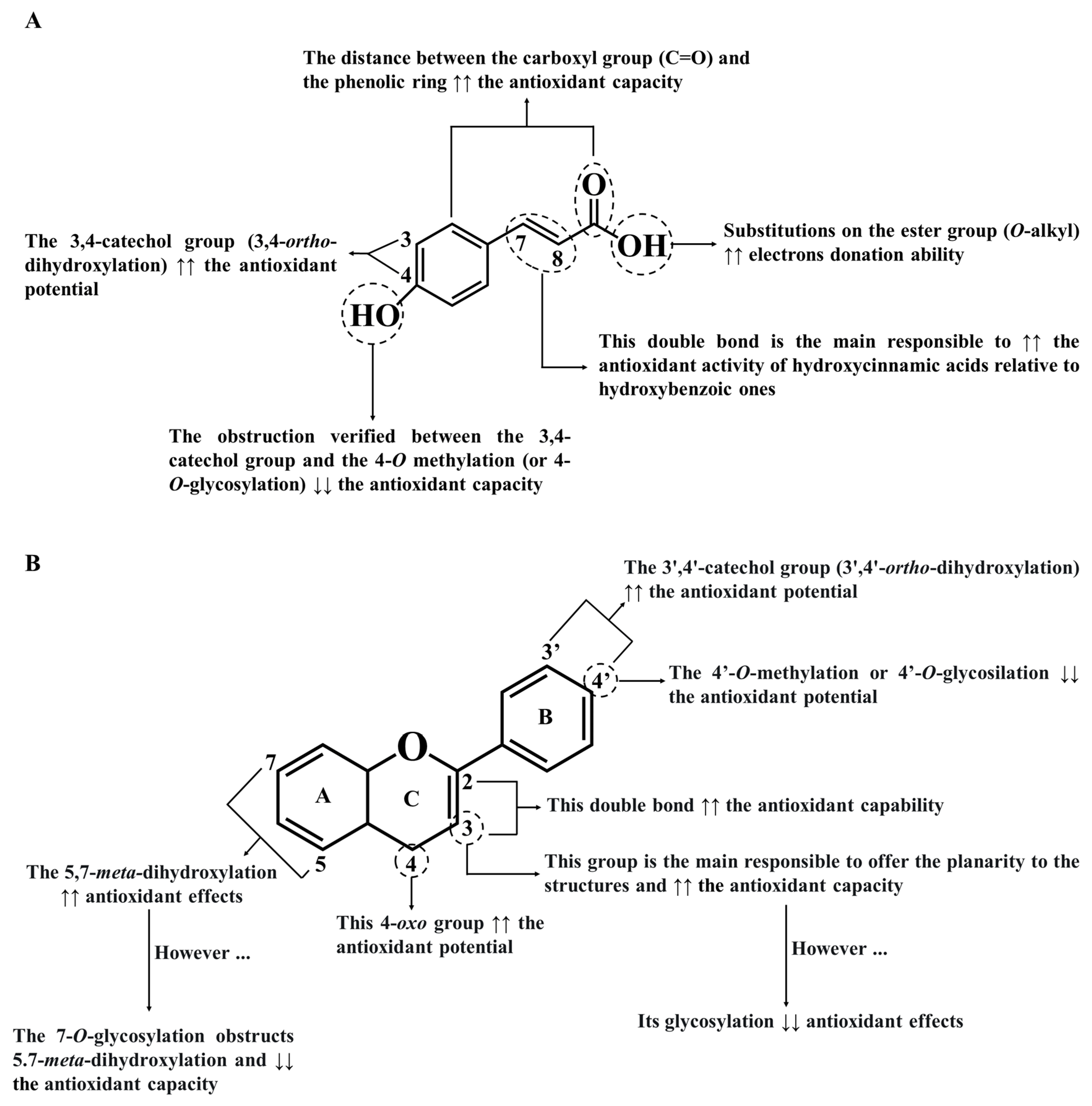 Figure 3. The main structure-activity relationship is responsible to influence the antioxidant capacity of phenolic acids (A) and flavonoids (B) (adapted from Bento et al. [5]).
Figure 3. The main structure-activity relationship is responsible to influence the antioxidant capacity of phenolic acids (A) and flavonoids (B) (adapted from Bento et al. [5]).The antioxidant activity and free radicals scavenging ability of flavonoids are stronger than those of phenolic acids because they have a higher number of hydroxyl groups [35]. In fact, the presence of (A) an o-diphenolic group and 3′,4′-catechol hydroxyl groups on B ring, (B) a double bond in positions 2 and 3, and (C) the conjugation between the double-bond and the 4-oxo group on C ring enhance the antioxidant properties of flavonoids when compared to other phenolic subclasses (Figure 3B) [5][40]. Therefore, it is not surprising that the absence of any of these features abolishes the antioxidant activity of phenolics. The various hydroxyl groups on the B ring confer the capacity to give hydrogen atoms and electrons to hydroxyl, peroxyl, and peroxynitrite radicals, stabilizing them [40]. Moreover, the hydroxyl group in carbon 3 of the C ring increases the antioxidant power of flavonoids, standing out the quercetin activity (TEAC value of 4.7 mM), which has almost twice the antioxidant potential of catechin (TEAC value of 2.4 mM) [43]. Another clear example is the fact that although taxifolin and quercetin have a 4-oxo group, the absence of the double bond between carbons 2 and 3 of taxifolin, makes it a weaker antioxidant agent than quercetin [44]. In addition, the 5,7-m-dihydroxy arrangement on the A ring also increases the antioxidant capabilities of flavonoids [43].
All of these findings explain the lower antioxidant activity of kaempferol compared to luteolin; although both have the same hydroxyl configuration, kaempferol does not have the catechol group on the B ring, and, therefore, is less effective [45]. Moreover, the presence of a free hydroxyl group at position 3 in flavan-3-ols and flavonols is an added value because it can originate intramolecular hydrogen bonds with the 3′,4′-catechol structure of the B ring, aligning it towards the A ring [46]. This torsion angle on the B ring concerning the rest of the molecule contributes to the planarity of the structure, promotes conjugation and electron dislocations, and thus reduces radical species amounts [45]. A good example of this evidence is quercetin, which effectively presents remarkable chelating and scavenging potential, while flavones and flavanones are less effective in reducing oxidative stress because they do not possess a 3-hydroxyl group in their structure [47]. On the other hand, conjugation with carbohydrate and glycoside residues on A and/or B rings decreases the antioxidant activities of flavonoids [40]. This explains why quercetin aglycone has a stronger scavenging potential towards peroxyl radicals and ferric species than its O-glycosylated derivatives [43][48]. Among the sugar substitutions, the glycosylation at position 4′ is more suppressive than the substitutions at positions 3 and 7 of the C- and A-rings, respectively, whereas the substitution at position 3 elicits a higher antioxidant potential than that at position 7 [49]. Finally, it has also been reported that the polymerization of flavonoids also raises their antioxidant potential [50]. For this reason, procyanidin dimers and trimers are more effective than monomeric flavonoids in reducing free radicals and reactive species [51].
Relatively to stilbenes and lignans, the relationship between structure and biological activity is not completely understood. Until now, it is certainly that, in stilbenes, the hydroxylation at position 4 and the increasing number of hydroxyl groups at the ortho position are added values, conferring more solubility and facilitating interaction with proteins [52]. Concerning methoxylated groups, they confer resistance to degradation; nevertheless, an excessive number of them may difficult target interactions [53]. Regarding lignans, their antioxidant potential is positively associated with the presence of catechol (3,4-dihydroxy phenyl) residues and the butanediol structure but a higher degree of oxidation at the benzylic positions diminish their ability to scavenge free radicals and reactive species [54][55].
Furthermore, it is also important to consider the O-methylation of hydroxyl groups also influences the planarity of phenolics; particularly, the obstruction of the 3′,4′-catechol group by 4′-O-methylation significantly compromises their scavenging effects. A clear example happens with quercetin: its 4′-O-methylation decreases abruptly the capacity to avoid ferrous sulphate-induced lipid peroxidation [56]. Similar effects were observed with the kaempferol-3′,4′-dimethylether, where its capacity to scavenge peroxyl radicals is reduced to about half when compared to kaempferol [57].
However, it is important to keep in mind that phenolics’ chemical structure, including their catechol and pyrogallol groups, makes them susceptible to autoxidation [58]. When a phenolic loses an electron or acts as a reducing agent, and becomes a radical, although it can be stable, its oxidized intermediates can acquire a pro-oxidant behaviour [58][59]. For example, the interaction between phenolics and transition metals can lead to pro-oxidant events that, at high concentrations, can cause side effects to human health [40]. For instance, it is interesting to note that the glycosylation and methylation of hydroxyl groups of flavonoids can weaken the pro-oxidative behaviour [57]. Additionally, pH can also influence phenolics antioxidant capacity [60]. In general, a lower pH increases iron-reducing activity but inhibits iron catalytic activity and reduces chelate properties. For instance, at pH 7.4, ρ-hydroxybenzoic acid shows scavenging potential, while apigenin 7-glucoside presents a pro-oxidative behaviour. However, both compounds at pH 5.8 do not have any antioxidant or pro-oxidant activity [61].
Considering the preceding, it is extremely important to understand the relationship between the chemical structure and the activity of phenolics in order to avoid their auto-oxidation and find the most promising ones.
2.2. Anti-Inflammatory Properties of Phenolics
Inflammation is part of the complex biological response of body tissues to harmful stimuli caused by injury, infection, toxins, or rash [31]. During this process occurs the release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukin (IL)-1β and IL-6, heat shock proteins, prostaglandins, and RNOS, including superoxide, nitric oxide, and hydrogen peroxide radicals [62]. When it is exaggerated, it triggers protein oxidation, damages carbohydrates, and nucleic acids and induces lipid peroxidation, and interferes with cellular functions, favouring the occurrence of several related ailments, such as metabolic problems, infections, rheumatoid arthritis, hypertension, and cancer [15]. Therefore, in uncontrolled situations, its removal is critical. Several efforts have been done to fully discover the anti-inflammatory capabilities of phenolics, being already reported that some of them can effectively relieve inflammatory responses, inhibiting expressions and/or activities of pro-inflammatory genes, including cyclooxygenases, angiotensin-converting enzyme, nuclear factor (NF)-κB, phosphoinositide 3-kinase/Akt (PI3 K/Akt) and mitogen-activated protein kinases (MAPKs) [38]. In addition, phenolics can activate p53, and hence, remove inflammation [63]. For example, ρ-coumaric acid showed a strong capacity to reduce TNF-α levels in a rat model of adjuvant-induced arthritis rats, whereas caffeic acid was able to inhibit lipopolysaccharide (LPS)-inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2 expression, and TNF-α and IL-1β levels in RAW 264.7 macrophages [64][65]. Caffeic acid and ellagic acid together at 2% also demonstrated the capacity to mitigate the production of IL-1β, IL-6, and TNF-α in diabetic mice [66]. By focusing on flavonoid compounds, coloured and non-coloured phenolics extracted from sweet cherries revealed the capability to inhibit oxide nitric production (IC50 scores of 338.31 and 367.93 µg/mL, respectively) and to suppress iNOS and COX-2 expression in LPS-activated murine macrophage RAW 264.7 cells [15], while daidzein, isorhamnetin, genistein, kaempferol, naringenin, and quercetin showed the capacity to suppress NF-κB and iNOS activation in murine J774 macrophages at 100 µM [62]. Kaempferol (0.02%) also revealed the ability to modulate the expression of NF-κB genes in older rats [67].
References
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93.
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726.
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397.
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637.
- Bento, C.; Gonçalves, A.C.; Jesus, F.; Simões, M.; Silva, L.R. Phenolic compounds: Sources, properties and applications. In Bioactive Compounds: Sources, Properties and Applications; Porter, R., Parker, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 271–299. ISBN 6312317269.
- Li, S.; Liu, J.; Li, Z.; Wang, L.; Gao, W.; Zhang, Z.; Guo, C. Sodium-dependent glucose transporter 1 and glucose transporter 2 mediate intestinal transport of quercetrin in Caco-2 cells. Food Nutr. Res. 2020, 64, 1–9.
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120.
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1; US Department of Agriculture: Beltsville, MD, USA, 2013. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav3-1.pdf (accessed on 26 November 2021).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Ock, K.C.; Sang, J.C.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252.
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989–1009.
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in food and methods of their determination. Foods 2020, 9, 645.
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90.
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688.
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Anti-inflammatory and antiproliferative properties of sweet cherry phenolic-rich extracts. Molecules 2022, 27, 268.
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535.
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923.
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312.
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315.
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15.
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2019, 70, 335–348.
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77.
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282.
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78.
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690.
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic compounds and bioaccessibility thereof in functional pasta. Antioxidants 2020, 9, 343.
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019.
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171.
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53.
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789.
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 2019, 24, e00370.
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263.
- Gonçalves, A.C.; Flores-Félix, D.; Costa, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Hepatoprotective effects of sweet cherry extracts (cv. Saco). Foods 2021, 10, 2623.
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346.
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213.
- Martinez-Negrin, G.; Acton, J.P.; Cocksedge, S.P.; Bailey, S.J.; Clifford, T. The effect of dietary (poly) phenols on exercise-induced physiological adaptations: A systematic review and meta-analysis of human intervention trials adaptations: A systematic review and meta-analysis of human intervention trials. Crit. Rev. Food Sci. Nutr. 2020, 1–16, 2872–2887.
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2010, 59, 1673–1685.
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584.
- Kalinowska, M.; Gołębiewska, E.; Swiderski, G.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Swisłocka, R. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in MDA-MB-231 and MCF-7 cell lines. Nutrients 2021, 13, 3107.
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 5666.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956.
- Ratty, A.K.; Das, N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: Structure-activity relationship. Biochem. Med. Metab. Biol. 1988, 39, 69–79.
- Van Acker, S.A.B.E.; De Groot, M.J.; van den Berg, D.-J.; Tromp, M.N.J.L.; Donné-Op den Kelder, G.; Van Der Vijgh, W.J.F.; Bast, A. A quantum chemical explanation of the antioxidant activity of flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312.
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure—Activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461.
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355.
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Quindry, J.C.; Hosick, P.A.; Hudson, M.H.; Still, L.; Henson, D.A.; Milne, G.L.; Morrow, J.D.; et al. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl. Physiol. Nutr. Metab. 2008, 33, 254–262.
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15.
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free Radic. Res. 1998, 29, 351–358.
- Rühlmann, A.; Antovic, D.; Müller, T.J.J.; Urlacher, V.B. Regioselective hydroxylation of stilbenes by engineered cytochrome P450 from Thermobifida fusca YX. Adv. Synth. Catal. 2017, 359, 984–994.
- Shi, Y.W.; Wang, C.P.; Liu, L.; Liu, Y.L.; Wang, X.; Hong, Y.; Li, Z.; Kong, L.D. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol. Nutr. Food Res. 2012, 56, 1433–1444.
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347.
- Yamauchi, S.; Hayashi, Y.; Nakashima, Y.; Kirikihira, T.; Yamada, K.; Masuda, T. Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J. Nat. Prod. 2005, 68, 1459–1470.
- Dugas, A.J.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure-activity relationships. J. Nat. Prod. 2000, 63, 327–331.
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760.
- Alov, P.; Tsakovska, I.; Pajeva, I. Computational studies of free radical-scavenging properties of phenolic compounds. Curr. Top. Med. Chem. 2015, 15, 85–104.
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340.
- Bayliak, M.M.; Burdyliuk, N.I.; Lushchak, V.I. Effects of pH on antioxidant and prooxidant properties of common medicinal herbs. Open Life Sci. 2016, 11, 298–307.
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870.
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on i. Mediators Inflamm. 2007, 2007, 045673.
- Maurya, A.K.; Vinayak, M. PI-103 and quercetin attenuate PI3K-AKT signaling pathway in T-cell lymphoma exposed to hydrogen peroxide. PLoS ONE 2016, 11, e0160686.
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176.
- Jung, W.K.; Choi, I.; Lee, D.Y.; Yea, S.S.; Choi, Y.H.; Kim, M.M.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; et al. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-κB pathways. Int. J. Biochem. Cell Biol. 2008, 40, 2572–2582.
- Chao, P.C.; Hsu, C.C.; Yin, M.C. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metab. 2009, 6, 33.
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.8K
Revisions:
2 times
(View History)
Update Date:
29 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




