
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Filipa Sousa | + 2012 word(s) | 2012 | 2020-09-25 08:44:19 | | | |
| 2 | Camila Xu | Meta information modification | 2012 | 2020-09-29 10:50:28 | | | | |
| 3 | Camila Xu | Meta information modification | 2012 | 2020-09-29 11:06:51 | | |
Video Upload Options
The high incidence of fungal infections has become a worrisome public health issue, having been aggravated by an increase in host predisposition factors. Despite all the drugs available on the market to treat these diseases, their efficiency is questionable, and their side effects cannot be neglected. Bearing that in mind, it is of upmost importance to synthetize new and innovative carriers for these medicines not only to fight emerging fungal infections but also to avert the increase in drug-resistant strains.
1. Definition
There is a wide range of fungal infections, from superficial, affecting skin, to systemic infections with invasion of internal organs [1]. Fungal infections affect millions of people every year worldwide. Of these, more or less 1.5 million are invasive fungal infections therefore requiring advanced treatment and hospitalization. Most of these disseminated infections are caused by Candida, Cryptococcus, Aspergillus, and Pneumocystis species, being the cause of cryptococcosis, candidiasis, aspergillosis, and pneumocystis pneumonia, respectively [2].
Superficial fungal infections are rather common and, despite rarely being life threatening, they can spread to other skin regions and even become widespread. Furthermore, they can be transmitted to other people and may cause secondary bacterial skin infections, harming the quality of a person’s life. Skin mycoses are classified according to the causative fungal agents into dermatophytosis, yeast infections, and mold infections [1].
2. Introduction
Invasive fungal infections represent a significant burden to healthcare systems, having high morbidity and mortality rates. These rates are most worrisome among immunocompromised patients that are more prone to opportunistic infections, such as patients with Acquired Immune Deficiency Syndrome (AIDS), transplant patients whose immune systems are suppressed to prevent organ rejection, patients with cancer who are taking immunosuppressive chemotherapy or autoimmune patients undergoing immunosuppressive therapy [2][3].
The currently major available agents to treat invasive fungal infections can be grouped into four main classes according to their mechanism of action: polyenes, azoles, allylamines, and echinocandins (Table 1) [4]. They all present drawbacks when it comes to spectrum of activity, drug–drug interactions, pharmacokinetics and pharmacodynamics, resistance mechanisms, and the toxicity of the compounds themselves. Furthermore, there are some limitations in terms of clinical efficacy and efficiency, mainly because of their physical-chemical properties, like their hydrophobic character that leads to a low solubility in water and also selectivity problems deriving from the similarities between fungi and human cells [3][5].
Table 1. Targets of each group of antifungals [6][7].
| Class | Target (Mechanism of Action) | Antifungal | |
|---|---|---|---|
| Azoles | Ergosterol (inhibition of lanosterol 14-α-demethylase) | Imidazoles | Miconazole |
| Econazole | |||
| Ketoconazole | |||
| Clotrimazole | |||
| Triazoles | Itraconazole | ||
| Fluconazole | |||
| Voriconazole | |||
| Allylamines | Ergosterol (inhibition of squalene epoxidase) | Terbinafine | |
| Naftifine | |||
| Butenafine | |||
| Polyenes | Cell membrane (production of ROS) | Amphotericin B | |
| Ergosterol (inhibition of lanosterol 14-α-demethylase) | Nystatin | ||
| Echinocandines | Cell wall (block of β-1,3 glucan synthesis) | Caspofungin, Micafungin, Anidulafungin | |
| Other antifungals | Chelation of polyvalent metal cations | Ciclopirox | |
| Microtubules (prevention of the formation of the mitotic spindle) | Griseofulvin | ||
| Ergosterol (inhibition of D14 reductase and D7-D8 isomerase) | Amorolfine | ||
Nevertheless, the design and development of new drug delivery systems or even new antifungals is an emerging need, owing to the following facts [8]:
-
There are 20–40% mortality rates with invasive mycoses, therefore these figures need to be improved;
-
The increase in patients undergoing prolonged antifungal therapies reflects the need to develop better fungicidal drugs and thus reduce the length of the treatments and the costs associated;
-
There is still space for improvement in pharmacokinetics and pharmacodynamics, in order to reduce the frequency of drug use;
-
More attention needs to be given to the host toxicities and drug–drug interactions of current therapy so that their effects can be eliminated or, at least, minimized;
-
New therapy groups with different mechanisms of action are needed; this way, these new drugs may synergize with present ones and allow better responses;
-
There is an alarming growth in antifungal resistance in all therapeutic groups [8].
Nanotechnology is an emerging field of science that has shown an undeniable versatility and has boosted a revolution when it comes to medical treatments, quicker diagnosis, cellular regeneration, and drug delivery [9][10]. The material to produce nanoparticles can be divided into three main groups: polymers, lipids, or metals, each one giving rise to a different type of nanoparticle [11]. The main representatives of each of these three different groups of nanoparticles are mentioned in Figure 1 below.
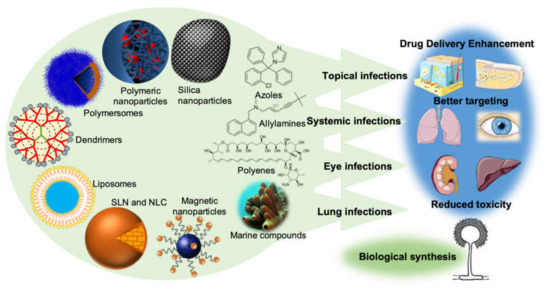
Figure 1. The new drug delivery systems based on nanotechnology that are currently being employed in order to enhance drug delivery, promote a better targeting, and reduce the toxicity of conventional antifungal drugs. It is also important to point out the importance of the production of nanoparticles by fungi (biological synthesis) and the undeniable potential of the sea as a source of new molecules with antifungal activity.
Nanoparticles have been employed in pharmaceutical formulations because of their ability to alter and improve the pharmacokinetic and pharmacodynamic properties of the drugs. This is given to their capability to increase the solubility and stability of the drugs, to allow a controlled release and to exhibit biocompatibility with tissues and cells, which is reflected in an overall improvement on therapeutic efficiency [11][12]. In addition, its subcellular size is compatible with an intravascular injection and its high surface area is amenable to modification so that the drug is released in a specific target, thus reducing the systemic adverse effects and increasing the therapeutic compliance, by decreasing the usual dose and the frequency of administration [13][14]. This targeted-specific action is possible since, at a nanomolecular level, it is possible to incorporate target ligands that allow a preferential binding of certain types of cells, by conjugation with antibodies and peptides on the surface of the transporters [15][16][17]. Hence, the development of new biopharmaceutical systems, especially nanoparticulate carriers, is a good strategy to improve the therapeutic efficacy, safety, and compliance of conventional antifungal drugs.
In Table 2 an overview of the new antifungal drug delivery systems is presented, and the drug chemical group, their route of administration, and their dosage form provided.
Table 2. Some of the novel drug delivery systems already developed for each antifungal drug.
| Antifungal Drugs | Novel Drug Delivery Systems | Routes of Administration | Dosage Forms | References |
|---|---|---|---|---|
| Miconazole | Niosomes | Transdermal | Gel | [18] |
| SLN | Oral | N.A. | [19] | |
| Topical | Gel | [20] | ||
| Microemulsion | Topical | N.A. | [21] | |
| Liposomes | Topical | Gel | [22] | |
| Nanoemulsion | Topical | N.A. | [23] | |
| Nanosponges | Vaginal | Gel | [24] | |
| Transfersomes | Topical | Gel | [25] | |
| Econazole | Microemulsion | Percutaneous | N.A. | [26] |
| Topical | Gel | [27] | ||
| SLN | Topical | Gel | [28] | |
| NLC | Topical | Gel | [29] | |
| Liposomes | Topical | Gel | [30] | |
| Ethosomes | Topical | Gel | [31] | |
| Transethosomes | Transdermal | Gel | [32] | |
| Nanosponges | Topical | Hydrogel | [33] | |
| Niosomes | Transdermal | Gel | [34] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| Nanoemulsion | Topical | N.A. | [36] | |
| Ketoconazole | SLN/NLC | Topical | Gel | [37] |
| Niosomes | Topical | Gel | [38] | |
| Microemulsion | Oral | N.A. | [39] | |
| Spanlastics | Ocular | N.A. | [40] | |
| Dendrimers | Topical | Hydrogel | [41] | |
| Liposomes | Topical | N.A. | [42] | |
| Clotrimazole | Liposomes | Topical | Gel | [43] |
| Nanosponges | Topical | Hydrogel | [44] | |
| Ethosomes | Topical | Gel | [45] | |
| Niosomes | Topical | Gel | [46] | |
| Polymeric emulgel | Topical | Gel | [47] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| SLN/NLC | Topical | N.A. | [48] | |
| Microemulsion | Buccal | Gel | [49] | |
| Vaginal | Gel | [24] | ||
| Transfersomes | Transdermal/Topical | N.A. | [50] | |
| Itraconazole | Transfersomes | Transdermal | N.A. | [51] |
| SLN | Ocular | N.A. | [52] | |
| NLC | Inhalation | N.A. | [53] | |
| Niosomes | Topical | N.A. | [54] | |
| Microemulsion | Transdermal | N.A. | [55] | |
| Liposomes | Topical | N.A. | [56] | |
| Polymeric nanoparticles | Oral | N.A. | [57] | |
| Polymersome | Intravenous | N.A. | [54] | |
| Spanlastics | Ocular | N.A. | [58] | |
| Silica nanoparticles | Oral | N.A. | [59] | |
| Fluconazole | Microemulsion | Vaginal | Gel | [60] |
| Niosomes | Ocular | Gel | [61] | |
| Liposomes | Intravitral | N.A. | [62] | |
| SLN | Topical | Gel | [63] | |
| NLC | Oral | N.A. | [64] | |
| Microsponges | Topical | Gel | [65] | |
| Ethosomes | Topical | Gel | [66] | |
| Spanlastics | Ocular | N.A. | [67] | |
| Polymeric amphiphilogel | Topical | Gel | [68] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| Voriconazole | Microemulsion | Ocular | N.A. | [69] |
| Polymeric nanoparticles | Ocular | N.A. | [70] | |
| Pulmonar | N.A. | [71] | ||
| SLN | Topical | Gel | [72] | |
| Transethosome | Topical | N.A. | [73] | |
| Ethosome | Topical | N.A. | [74] | |
| Terbinafine | Liposomes | Topical | Gel | [75] |
| SLN | Topical | N.A. | [76] | |
| Transfersomes | Topical | N.A. | [77] | |
| Spanlastics | Transungual | N.A. | [78] | |
| Polymeric chitosan nanoparticles | Topical | Hydrogel | [79] | |
| Naftifine | Microemulsion | Topical | N.A. | [80] |
| Niosomes | Topical | Gel | [81] | |
| Butenafine | Microemulsion | Topical | Hydrogel | [82] |
| Amphotericin B | Liposomes | Intravenous | N.A. | [83] |
| SLN/NLC | Oral | N.A. | [84] | |
| Topical | N.A. | [85] | ||
| Magnetic nanoparticles | Nasal instilation | N.A. | [86] | |
| Nanoemulsion | Topical | N.A. | [87] | |
| Polymeric nanoparticles | Intravenous | N.A. | [88] | |
| Oral | N.A. | [89] | ||
| Polymersomes | Oral | N.A. | [90][91] | |
| Transfersomes | Topical | N.A. | [92] | |
| Micelles | Intravenous | N.A. | [93] | |
| Silica nanoparticles | Intravenous | N.A. | [94] | |
| Nystatin | SLN | Topical | N.A. | [95] |
| Nanoemulsion | Topical | N.A. | [96] | |
| Liposomes | Intravenous | N.A. | [97] | |
| Niosomes | Parenteral | N.A. | [98] | |
| Griseofulvin | Niosomes | Oral | N.A. | [99] |
| Ciclopirox | Niosomes | Topical | Gel | [100] |
| Caspofungin, Micafungin, Anidulafungin, Amorolfine | No nano-tech studies yet released | |||
N.A.: the dosage form is not mentioned in the reference cited; SLN: Solid Lipid Nanoparticles; NLC: Nanostructured Lipid Carriers.
However, the efficacy and human safety of these new therapies remain uncertain in most of the articles found in literature. They generally lack controlled clinical trials and sometimes the suggested routes of administration are less practical, or the production cost may hinder the replacement of the conventional treatment. Nevertheless, in other cases, the opposite is verified, and some options have potential to become a viable first line treatment [101]. Moreover, given the widespread use of antifungal agents and the limited therapeutic offer, fungi have developed resistance mechanisms, like overexpression of efflux pump proteins and formation of biofilms. These mechanisms can mean not only a decrease in a drug’s effective concentration, but also changes and subexpression of drug targets and metabolic bypass [6]. It is important to add that resistance is a cross-cutting issue to all of the currently available classes of antifungal agents, therefore overcoming antifungal resistance can be considered as the mainstay for improving therapeutic strategies to treat antifungal infections [2][102].
Despite the uprising of these issues in antifungal therapy, there are several mechanisms by which nanoparticles overcome the development of resistance mechanisms:
-
The chemical features and simultaneous multiple mechanisms used by nitric oxide, chitosan, and metallic nanoparticles make the likelihood of resistance development unviable (for example, through the direct reaction of reactive nitrogen oxide intermediates with DNA structure)[103] [104];
-
The resistance mechanisms can be prevented by packaging multiple antimicrobial drugs within the same nanoparticle, because the likelihood of multiple simultaneous gene mutations in the same cell is low. The most striking examples are the encapsulation of antifungal drugs in chitosan or silver nanoparticles, combining the antifungal properties of both and decreasing the possibility of drug resistance [103][105];
-
Some nanoparticles, such as liposomes and dendrimers, are able to overcome the resistance mechanisms of decreased uptake and increased efflux of drug from the microbial cell. Liposomes are able to quickly fuse with the plasma membrane of the microbial cell and release a high concentration of drug into its plasma membrane or cytoplasm, thereby circumventing the decreased uptake mechanism of resistance. This means a faster delivery and avoidance of the transmembrane pumps that catalyze increased efflux of drugs. Dendrimers, on the other hand, are extensively branched molecules, whose surface can be filled with positively charged quaternary ammonium compounds, which bind to negatively charged microbial cell envelopes and increase membrane permeability. This allows the entrance of more dendrimers to the microbial cell, the flow of its cytoplasmic contents to the exterior, and the ultimate destruction of the microbial cell membrane. This goes to show that dendrimers are also able to surpass the resistance mechanism of decreased uptake of drug [106]. Other nanoparticles, specifically nitric oxide nanoparticles made of silica and zinc oxide nanoparticles are able to overcome biofilm formation by killing the microbes present in already formed biofilms or by inhibiting biofilm formation through the generation of reactive oxygen species, respectively [107][108];
-
Nanoparticles have been used to target antifungal drugs to the specific site of infection, allowing the local release of high concentrations of drug, while keeping the total dose of drug administered low. This high local dose is able to destroy the infecting fungi before they can develop resistance, thereby overcoming this worrisome issue and translating into fewer side effects upon the patient [103].
That being said, it is also important that the research done, not only focuses on formulating these systems, but also in overcoming the major challenges that their placing on the market faces: the physical instability of nanoparticles, their small capacity of drug loading, the cytotoxicity/immunogenicity, and the high cost of production and standardization, given the complexity of the formulations. Besides that, there is almost a complete lack of studies in vivo as reaching the therapeutic range needed to perform these studies has proven to be an arduous job. That lies in the fact that, in many cases, there is an anticipated release of the drug, aggregation and precipitation of the nanoparticles, and the accumulation in non-target tissues.
References
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Anasane, N.; Dolenc-Voljč, M. Nanotechnology for the Treatment of Fungal Infections on Human Skin. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Academic Press: Cambridge, MA, USA, 2017; pp. 169–184. [Google Scholar]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Chang, Y.L.; Yu, S.J.; Heitman, J.; Wellington, M.; Chen, Y.L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Morovati, H.; Aghebati-Maleki, L. Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed. Pharm. 2019, 110, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Sirish Sadhna Khatry, S.N.; Sadanandan, M. Novel Drug Delivery Systems for Antifungal Therapy. Int. J. Pharm. Pharm. Sci. 2010, 2, 6–9. [Google Scholar]
- Perfect, J.R. Is there an emerging need for new antifungals? Expert Opin Emerg Drugs 2016, 21, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Roli Jain, A.P. A Review of Kinetics of Nanoparticulated Delayed Release Formulations. J. Nanomed. Nanotechnol. 2015, 6, 2. [Google Scholar]
- Hasan, S. A review on Nanoparticles: Their Synthesis and Types. Res. J. Recent Sci. 2015, 4, 1–3.
- Nagavarma, B.V.N.; Ayaz, A.H.K.S.Y.; Vasudha, L.S.; Shivahumar, H.G. Different Techniques for Preparation of Polymeric Nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed Paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2017, 536, 95–107. [Google Scholar] [CrossRef]
- Jinhyun Hannah Lee, Y.Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 12. [Google Scholar]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Rangari, A.T. Polymeric Nanoparticles Based Topical Drug Delivery: An Overview. Asian J. Biomed. Pharm. Sci. 2015, 5, 5–12. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Chapter 2—Overview of Controlled Release Mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery, Advances in Delivery Science and Technology; Society, C.R., Ed.; Springer: New York, NY, USA, 2012.
- Kumar, A.S.; Sheri, P.S.; Kuriachan, M.A. Formulation and Evaluation of Antifungal Nanosponge Loaded Hydrogel for Topical Delivery. Int. J. Pharm. Pharm. Res. 2018, 13, 362–379. [Google Scholar]
- Akhtar, N.; Pathak, K. Cavamax W7 Composite Ethosomal Gel of Clotrimazole for Improved Topical Delivery: Development and Comparison with Ethosomal Gel. Am. Assoc. Pharm. Sci. 2012, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Shirsand, S.; Kumar, R.; Keshavshetti, G.; Bushetti, S.S.; Padivala, V.S. Formulation and Evaluation of Clotrimazole Niosomal Gel for Topical Application. Rajiv Gandhi Univ. Health Sci. J. Pharm. Sci. 2015, 5, 32–38. [Google Scholar] [CrossRef]
- Yassin, G.E. Formulation and Evaluation of Optimized Clotrimazole Emulgel Formulations. Br. J. Pharm. Res. 2014, 4, 1014–1030. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Kaewbanjong, J.; Heng, P.W.S.; Boonme, P. Clotrimazole microemulsion and microemulsion-based gel: Evaluation of buccal drug delivery and irritancy using chick chorioallantoic membrane as the model. J. Pharm. Pharm. 2017, 69, 1716–1723. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion-based vaginal gel of clotrimazole: Formulation, in vitro evaluation, and stability studies. AAPS Pharmscitech 2009, 10, 476–481.
- Firthouse, P.U.M.; Halith, S.M.; Wahab, S.U.; Sirajudeen, M.; Mohideen, S.K. Formulation and Evaluation of Miconazole Niosomes. Int. J. Pharmtech Res. 2011, 3, 1019–1022. [Google Scholar]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Pokharkar, V.; Madgulkar, A.; Patil, N.; Patil, N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharmscitech 2009, 10, 289–296. [Google Scholar] [CrossRef]
- Shahzadi, I.; Masood, M.I.; Chowdhary, F.; Anjum, A.A.; Nawaz, M.A.; Maqsood, I.; Zaman, M.Q. Microemulsion Formulation for Topical Delivery of Miconazole Nitrate. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 30–36. [Google Scholar]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: Comparison with conventional liposomes. AAPS Pharmscitech 2012, 13, 723–731. [Google Scholar] [CrossRef]
- Maha, H.L.; Sinaga, K.R.; Masfria, M. Formulation and evaluation of miconazole nitrate nanoemulsion and cream. Asian J. Pharm. Clin. Res. 2018, 11, 319–321. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hematheerthani, N.; Ratna, J.V.; Saikishore, V. Design and characterization of miconazole nitrate loaded nanosponges containing vaginal gels. Int. J. Pharm. Anal. Res. 2016, 5, 410–417. [Google Scholar]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, Optimization and Characterization of a Transfersomal Gel Using Miconazole Nitrate for the Treatment of Candida Skin Infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Ge, S.; Lin, Y.; Lu, H.; Li, Q.; He, J.; Chen, B.; Wu, C.; Xu, Y. Percutaneous delivery of econazole using microemulsion as vehicle: Formulation, evaluation and vesicle-skin interaction. Int. J. Pharm. 2014, 465, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Evelyn, D.; Wooi, C.C.; Kumar, J.R.; Muralidharan, S.; Dhanaraj, S.A. Development and evaluation of microemulsion based gel (MBGs) containing econazole nitrate for nail fungal infection. J. Pharm. Res. 2012, 5, 2385–2390. [Google Scholar]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharm. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Keshri, L.; Pathak, K. Development of thermodynamically stable nanostructured lipid carrier system using central composite design for zero order permeation of econazole nitrate through epidermis. Pharm. Dev. Technol. 2013, 18, 634–644. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Transethosomes of Econazole Nitrate for Transdermal Delivery: Development, In-vitro Characterization, and Ex-vivo Assessment. Pharm. Nanotechnol. 2018, 6, 171–179. [Google Scholar] [CrossRef]
- Sharma, R.; Walker, R.B.; Pathak, K. Evaluation of the Kinetics and Mechanism of Drug Release from Econazole nitrate Nanosponge Loaded Carbapol Hydrogel. Indian J. Pharm. Educ. Res. 2011, 45, 25–31. [Google Scholar]
- Kumar, Y.P.; Kumar, K.V.; Shekar, R.R.; Ravi, M.; Kishore, V.S. Formulation and Evaluation of Econazole Niosomes. Sch. Acad. J. Pharm. 2013, 2, 315–318. [Google Scholar]
- Bachhav, Y.G.; Mondon, K.; Kalia, Y.N.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Youenang Piemi, M.P.; Korner, D.; Benita, S.; Jean-Paul, M. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J. Control. Release 1999, 58, 177–187. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. SLN and NLC for topical delivery of ketoconazole. J. Microencapsul. 2005, 22, 501–510. [Google Scholar] [CrossRef]
- Shirsand, S.; Para, M.; Nagendrakumar, D.; Kanani, K.; Keerthy, D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int. J. Pharm. Investig. 2012, 2, 201–207. [Google Scholar] [CrossRef]
- Tiwari, N.; Sivakumar, A.; Mukherjee, A.; Chandrasekaran, N. Enhanced antifungal activity of Ketoconazole using rose oil based novel microemulsion formulation. J. Drug Deliv. Sci. Technol. 2018, 47, 434–444. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Ashe, S.; Nayak, D.; Tiwari, G.; Rauta, P.R.; Nayak, B. Development of liposome-encapsulated ketoconazole: Formulation, characterisation and evaluation of pharmacological therapeutic efficacy. Micro. Nano Lett. 2015, 10, 126–129. [Google Scholar] [CrossRef]
- Ning, M.; Guo, Y.; Pan, H.; Chen, X.; Gu, Z. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev. Ind. Pharm. 2005, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.G.S.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. Spj. Off. Publ. Saudi Pharm. Soc. 2012, 20, 161–170.
- Zheng, W.S.; Fang, X.Q.; Wang, L.L.; Zhang, Y.J. Preparation and quality assessment of itraconazole transfersomes. Int. J. Pharm. 2012, 436, 291–298.
- Mohanty, B.; Majumdar, D.K.; Mishra, S.K.; Panda, A.K.; Patnaik, S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 2015, 20, 458–464.
- Pardeike, J.; Weber, S.; Haber, T.; Wagner, J.; Zarfl, H.P.; Plank, H.; Zimmer, A. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int. J. Pharm. 2011, 419, 329–338.
- Wagh, V.D.; Deshmukh, O.J. Itraconazole Niosomes Drug Delivery System and Its Antimycotic Activity against Candida albicans. Isrn. Pharm. 2012, 2012, 653465.
- Chudasama, A.; Patel, V.; Nivsarkar, M.; Vasu, K.; Shishoo, C. Investigation of microemulsion system for transdermal delivery of itraconazole. J. Adv. Pharm. Technol. Res. 2011, 2, 30–38. [Google Scholar]
- Leal, A.F.; Leite, M.C.; Medeiros, C.S.; Cavalcanti, I.M.; Wanderley, A.G.; Magalhaes, N.S.; Neves, R.P. Antifungal activity of a liposomal itraconazole formulation in experimental Aspergillus flavus keratitis with endophthalmitis. Mycopathologia 2015, 179, 225–229. [Google Scholar] [CrossRef]
- Leal, A.F.; Leite, M.C.; Medeiros, C.S.; Cavalcanti, I.M.; Wanderley, A.G.; Magalhaes, N.S.; Neves, R.P. Development of an itraconazole encapsulated polymeric nanoparticle platform for effective antifungal therapy. J. Mater. Chem. B 2016, 4, 1787–1796. [Google Scholar]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [CrossRef]
- Mellaerts, R.; Mols, R.; Jammaer, J.A.G.; Aerts, C.A.; Annaert, P.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P.; Martens, J.A. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur. J. Pharm. Biopharm. 2008, 69, 223–230. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm. 2009, 365, 175–179. [Google Scholar] [CrossRef]
- Soliman, O.A.E.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro-in vivo evaluation. Pharm. Dev. Technol. 2017, 24, 1–52. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dhingra, N.; Velpandian, T.; Jaiswal, J. Efficacy of fluconazole and liposome entrapped fluconazole for C. albicans induced experimental mycotic endophthalmitis in rabbit eyes. Acta Ophthalmol. Scand 2000, 78, 448–450. [Google Scholar] [CrossRef] [PubMed]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2017, 25, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Moazeni, M.; Babaei, R.; Saeedi, M.; Akbari, J.; Parkoohi, P.I.; Nabili, M.; Morteza-Semnani, A.A.G.K.; Nokhodchi, A. Improved Yeast Delivery of Fluconazole with a Nanostructured Lipid Carrier System. Biomed. Pharmacother. 2017, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Deb, T.K.; Osmani, R.A.M.; Bhosale, R.R.; Hani, U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J. Basic Clin. Pharm. 2016, 7, 39–48. [Google Scholar] [PubMed]
- Indora, N.; Kaushik, D. Design, development and evaluation of ethosomal gel of fluconazole for topical fungal infection. Int. J. Eng. Sci. Invent. Res. Dev. 2015, 1, 280–306. [Google Scholar]
- Kaur, I.P.; Rana, C.; Singh, M.; Bhushan, S.; Singh, H.; Kakkar, S. Development and evaluation of novel surfactant-based elastic vesicular system for ocular delivery of fluconazole. J. Ocul. Pharm. 2012, 28, 484–496. [Google Scholar] [CrossRef]
- Lalit, S.K.; Panwar, A.S.; Darwhekar, G.; Jain, D.K. Formulation and Evaluation of Fluconazole Amphiphilogel. Der. Pharm. Lett. 2011, 3, 125–131. [Google Scholar]
- Kumar, R.; Sinha, V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef]
- Basaran, E.; Karaca, H.; Yenilmez, E.; Guven, U. Voriconazole incorporated polymeric nanoparticles for ocular application. Lat. Am. J. Pharm. 2017, 36, 1983–1994. [Google Scholar]
- Das, P.J.; Paul, P.; Mukherjee, B.; Mazumder, B.; Mondal, L.; Baishya, R.; Debnath, M.C.; Dey, K.S. Pulmonary Delivery of Voriconazole Loaded Nanoparticles Providing a Prolonged Drug Level in Lungs: A Promise for Treating Fungal Infection. Mol. Pharm. 2015, 12, 2651–2664. [Google Scholar] [CrossRef]
- Pandurangan, D.K.; Bodagala, P.; Palanirajan, V.K.; Govindaraj, S. Formulation and evaluation of voriconazole ophthalmic solid lipid nanoparticles in situ gel. Int. J. Pharm. Investig. 2016, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2011, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome. Res. 2018, 28, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, S.T.; Hilmioglu Polat, S.; Yesim Metin, D.; Kandiloglu, G.; Ozer, O. Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: In vitro and in vivo evaluation. J. Liposome Res. 2016, 26, 163–173. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, D.-Z.; Liu, J.-J.; Chang, T.-W.; Ho, H.-O.; Sheu, M.-T. Development of terbinafine solid lipid nanoparticles as a topical delivery system. Int. J. Nanomed. 2012, 7, 4409–4418. [Google Scholar]
- Ghannoum, M.; Isham, N.; Henry, W.; Kroon, H.A.; Yurdakul, S. Evaluation of the morphological effects of TDT 067 (terbinafine in Transfersome) and conventional terbinafine on dermatophyte hyphae in vitro and in vivo. Antimicrob. Agents Chemother. 2012, 56, 2530–2534. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. Aaps Pharmscitech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Ozcan, I.; Abaci, O.; Uztan, A.H.; Aksu, B.; Boyacioglu, H.; Guneri, T.; Ozer, O. Enhanced topical delivery of terbinafine hydrochloride with chitosan hydrogels. Aaps Pharmscitech 2009, 10, 1024–1031. [Google Scholar] [CrossRef]
- Erdal, M.S.; Ozhan, G.; Mat, M.C.; Ozsoy, Y.; Gungor, S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: Characterization studies and in vitro and in vivo evaluations. Int. J. Nanomed. 2016, 11, 1027–1037. [Google Scholar] [CrossRef]
- Barakat, H.S.; Darwish, I.A.; El-Khordagui, L.K.; Khalafallah, N.M. Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev. Ind. Pharm. 2009, 35, 631–637. [Google Scholar] [CrossRef]
- Pillai, A.B.; Nair, J.V.; Gupta, N.K.; Gupta, S. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Derm. Res. 2015, 307, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J. Liposomal amphotericin B, AmBisome. J. Infect. 1994, 28 (Suppl. 1), 35–43. [Google Scholar] [CrossRef]
- Jansook, P.; Pichayakorn, W.; Ritthidej, G.C. Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): Effect of drug loading and biopharmaceutical characterizations. Drug Dev. Ind. Pharm. 2018, 44, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf. B Biointerfaces 2016, 139, 17–24. [Google Scholar] [CrossRef]
- Saldanha, C.A.; Garcia, M.P.; Iocca, D.C.; Rebelo, L.G.; Souza, A.C.; Bocca, A.L.; Almeida Santos Mde, F.; Morais, P.C.; Azevedo, R.B. Antifungal Activity of Amphotericin B Conjugated to Nanosized Magnetite in the Treatment of Paracoccidioidomycosis. PLoS Negl. Trop Dis. 2016, 10, e0004754. [Google Scholar] [CrossRef]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef]
- Souza, A.C.; Nascimento, A.L.; de Vasconcelos, N.M.; Jeronimo, M.S.; Siqueira, I.M.; R-Santos, L.; Cintra, D.O.; Fuscaldi, L.L.; Pires Junior, O.R.; Titze-de-Almeida, R.; et al. Activity and in vivo tracking of Amphotericin B loaded PLGA nanoparticles. Eur. J. Med. Chem. 2015, 95, 267–276. [Google Scholar] [CrossRef]
- Italia, J.L.; Kumar, M.N.; Carter, K.C. Evaluating the potential of polyester nanoparticles for per oral delivery of amphotericin B in treating visceral leishmaniasis. J. Biomed. Nanotechnol. 2012, 8, 695–702. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, H.; Sun, L.; Hou, W.; Cai, S.; Zhang, R.; Liu, F. Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. Int. J. Nanomed. 2014, 9, 5403–5413. [Google Scholar]
- Jay Prakash Jain, N.K. Development of amphotericin B loaded polymersomes based on (PEG)3-PLA co-polymers: Factors affecting size and in vitro evaluation. Eur. J. Pharm. Sci. 2010, 40, 456–465. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodriguez, A.C.; Torrado-Duran, S.; Molero, G.; Garcia-Rodriguez, J.J.; Torrado-Santiago, S. Efficacy and toxicity evaluation of new amphotericin B micelle systems for brain fungal infections. Int. J. Pharm. 2015, 494, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.; Gaidul, K.; Goldina, I.; Konenkov, V.; Kozlov, V.; Lyakhov, N.; Dushkin, A. Silica Nanoparticles as a Basis for Efficacy of Antimicrobial Drugs. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 551–575. [Google Scholar]
- Khalil, R.M.; Rahman, A.A.A.E.; Kassem, M.A.; Ridi, M.S.E.; Samra, M.M.A.; Awad, G.E.A.; Mansy, S.S. Preparation and in vivo Assessment of Nystatin-Loaded Solid Lipid Nanoparticles for Topical Delivery against Cutaneous Candidiasis. Int. J. Pharmacol. Pharm. Sci. 2014, 8, 401–409. [Google Scholar]
- Fernandez-Campos, F.; Clares Naveros, B.; Lopez Serrano, O.; Alonso Merino, C.; Calpena Campmany, A.C. Evaluation of novel nystatin nanoemulsion for skin candidosis infections. Mycoses 2013, 56, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Offner, F.; Krcmery, V.; Boogaerts, M.; Doyen, C.; Engelhard, D.; Ribaud, P.; Cordonnier, C.; de Pauw, B.; Durrant, S.; Marie, J.P.; et al. Liposomal nystatin in patients with invasive aspergillosis refractory to or intolerant of amphotericin B. Antimicrob. Agents Chemother. 2004, 48, 4808–4812. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Abdelbary, A.; Essam, T.; Abd El-Salam, R.M.; Aly Kassem, A.A. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin. Drug Dev. Ind. Pharm. 2011, 37, 1491–1508. [Google Scholar] [CrossRef]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. Aaps Pharmscitech 2009, 10, 1186–1192.
- Shirsand, S.B.; Keshavshetti, G.G. Formulaiton and characterization of drug loaded niosomes for antifungal activity. Sper. J. Adv. Nov. Drug Deliv. 2016, 1, 12–17. [Google Scholar]
- Jaya raja Kumar, S.M.; Subramani, P. Antifugal Agents: New Approach for Novel Delivery Systems. J. Pharm. Sci. Res. 2014, 6, 229–235. [Google Scholar]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.P.; Hu, C.-M.J.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594.
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511.
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.P.; Hu, C.-M.J.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594.
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511.





