Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bibhuti B Das | -- | 2159 | 2022-04-23 06:01:45 | | | |
| 2 | Bibhuti B Das | -11 word(s) | 2148 | 2022-04-23 06:07:35 | | | | |
| 3 | Peter Tang | Meta information modification | 2148 | 2022-04-24 04:09:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Das, B. Patent Foramen Ovale. Encyclopedia. Available online: https://encyclopedia.pub/entry/22193 (accessed on 03 March 2026).
Das B. Patent Foramen Ovale. Encyclopedia. Available at: https://encyclopedia.pub/entry/22193. Accessed March 03, 2026.
Das, Bibhuti. "Patent Foramen Ovale" Encyclopedia, https://encyclopedia.pub/entry/22193 (accessed March 03, 2026).
Das, B. (2022, April 23). Patent Foramen Ovale. In Encyclopedia. https://encyclopedia.pub/entry/22193
Das, Bibhuti. "Patent Foramen Ovale." Encyclopedia. Web. 23 April, 2022.
Copy Citation
A patent foramen ovale (PFO) is a commonly discovered potential opening between the right atrium (RA) and left atrium (LA) on routine echocardiographic surveillance in an otherwise asymptomatic infant or child. The importance of a PFO in some critical congenital heart defects (CHD), especially in neonates, is well recognized.
foramen ovale
patent foramen ovale (PFO)
1. Introduction
A patent foramen ovale (PFO) is a commonly discovered potential opening between the right atrium (RA) and left atrium (LA) on routine echocardiographic surveillance in an otherwise asymptomatic infant or child (Figure 1A,B). Screening for a PFO in an asymptomatic patient or follow-up for an isolated PFO is not justified [1]. There are limited data on the underlying causes of a PFO, although there is some good evidence that genetic factors play a role [2]. The prevalence of PFO in the general population is 25% and the median diameter of a PFO is 5 mm (mean ± standard deviation, 4.9 ± 2.6 mm); very rarely, a PFO can enlarge on follow-up [3][4]. There has been much discourse about the differences between a benign PFO and a pathological secundum atrial septal defect (ASD). The criteria to differentiate these two are the size of the hole, the presence or absence of a flap of septal tissue and the size of the interatrial shunt [5].
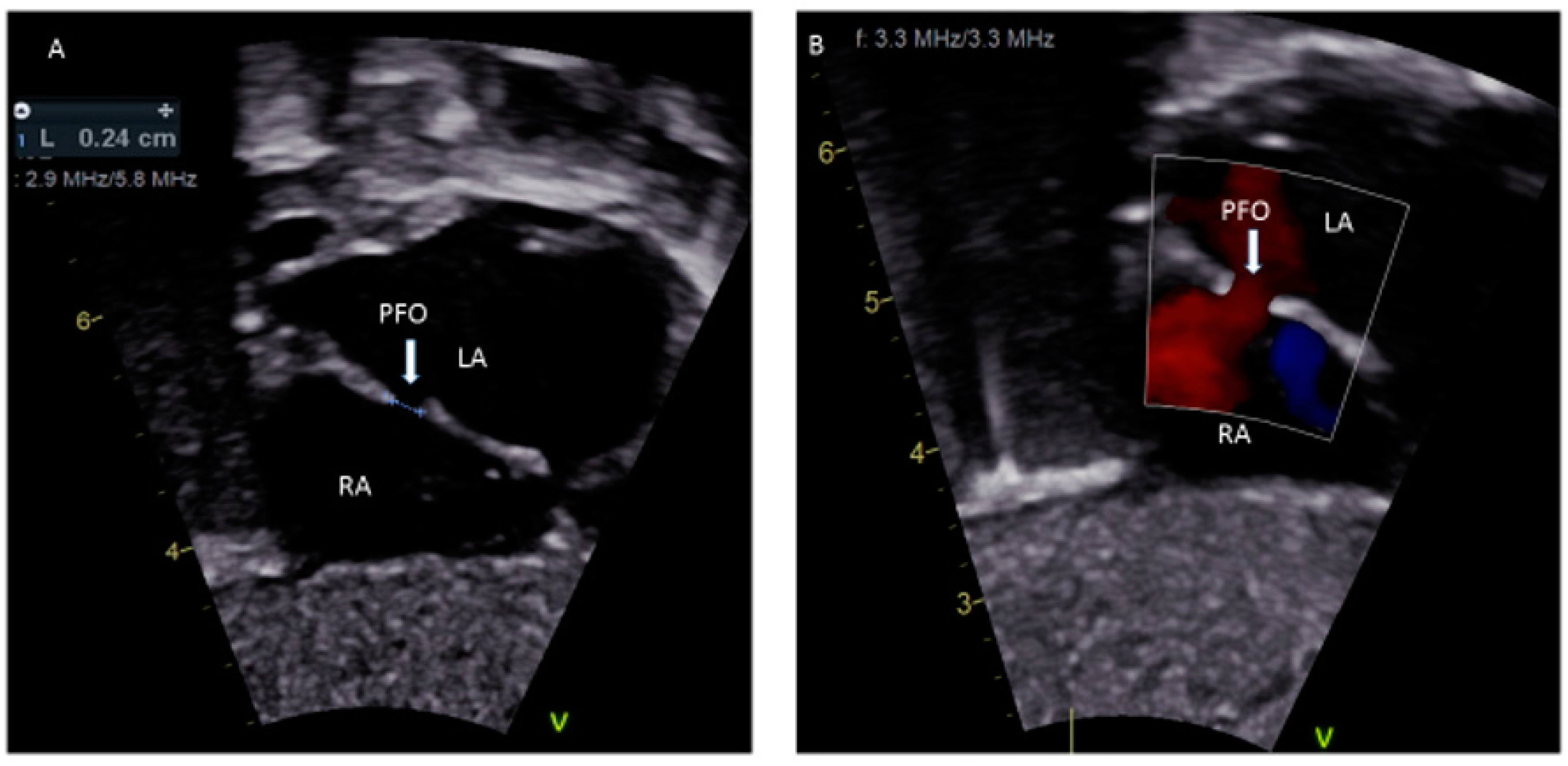
Figure 1. (A) Sub-costal view showing a 2.4 mm PFO; (B) Color Doppler demonstrating left to right shunting.
2. PFO in Fetal Life
In the embryonic stage, between 4 and 5 weeks of gestation, the septum primum begins to grow from the posterosuperior region of the primitive common atrium and grows in the midline towards the endocardial cushions at the crux of the heart. Smaller fenestrations develop in the mid-portion of the septum primum, even as the most inferior part merges with the developing endocardial cushions. The septum secundum also arises from the posterosuperior region of the common atrial wall, just to the right of the septum primum, and grows towards the endocardial cushions. Its inferior edge is concave and forms the crista dividens (limbus of the fossa ovalis). A second orifice forms as a flap valve mechanism in the upper portion of the septum primum (Figure 2A), which remains patent during fetal life as an FO (Figure 2B).
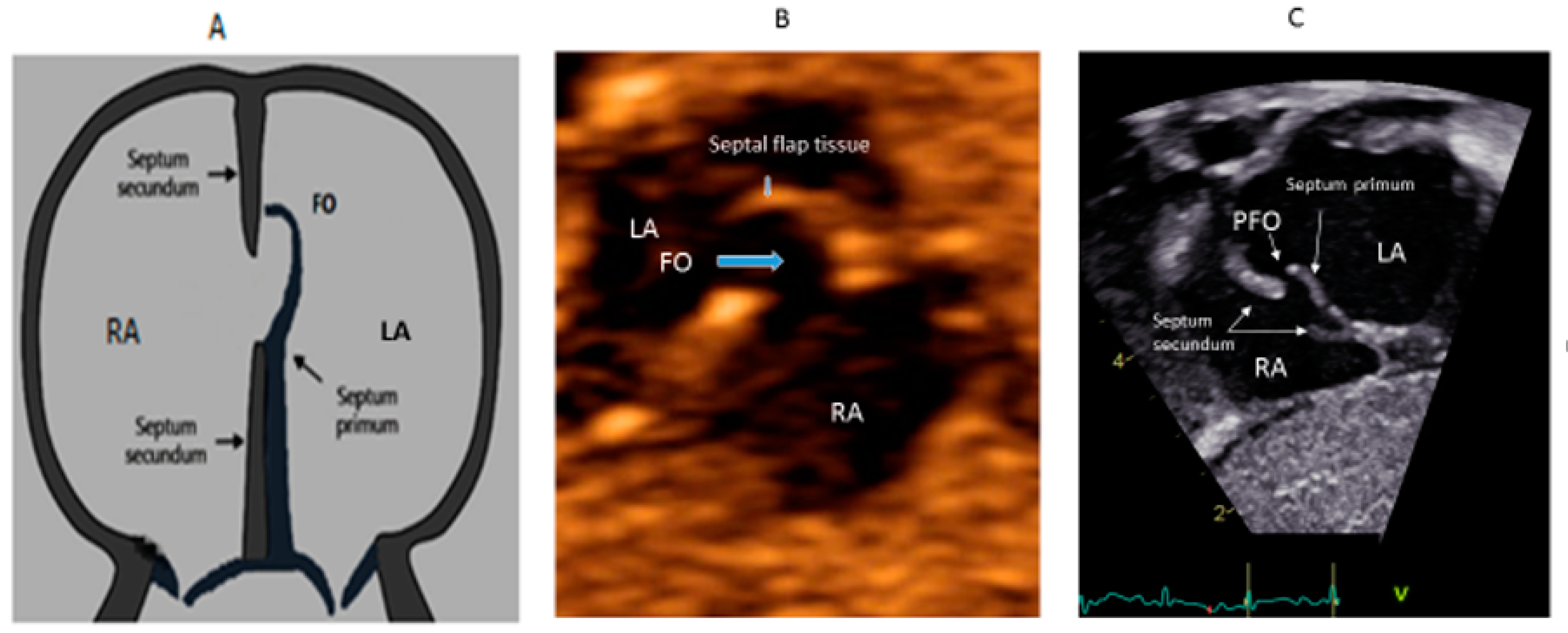
Figure 2. Correlation between development of atrial septum (A), Fetal (B) and post-natal anatomy (C).
During fetal life, the Eustachian valve on the leftward ridge of the inferior vena cava facilitates the shunting of oxygenated blood from the umbilical vein to the left atrium (LA) via FO. This allows more oxygenated blood to return to the left ventricle (LV). This physiological right to left shunt in fetal life is large, unobstructed, and essential [6]. At birth, the instant increase in pulmonary blood flow due to the onset of spontaneous ventilation is associated with increased venous return to LA. The distension of the LA with higher volume, along with the decreased vena caval flow into RA after cord clamping, results in the movement of the primum septum rightwards and allows the apposition and subsequent fusion of the primum with the secundum membranes. A deficiency in the membranous portion of the primum and the limbus results in a PFO (Figure 2C).
The FO in fetal life can be reliably demonstrated by echocardiography using ultrasound modalities such as 2D, color, and spectral Doppler during the second half of pregnancy [7]. The fetal FO is however difficult to demonstrate circumferentially despite advances in ultrasound imaging; hence, the transverse diameter is commonly used [7]. Fetal premature closure or restriction of the FO has been reported at any gestational age with variable effects on fetal hemodynamics and adverse fetal and postnatal outcomes. The spectrum of clinical presentation is related to the gestational age when the restriction first occurs and the severity of the restriction. Significant flow restriction results in an additional amount of blood being re-directed to the right ventricle (RV) as it bypasses the LA, causing right heart failure manifesting as pleural/pericardial effusions, ascites, necrotizing enterocolitis, and non-immune hydrops fetalis. There is also an association between the premature closure of the FO with neonatal mortality and complications such as prematurity, maternal preeclampsia, and placental abruption [8].
3. PFO in Neonates and Infants
The hemodynamic changes after birth lead to a smooth transition of fetal circulation and primarily depend upon the drop in pulmonary vascular resistance (PVR) and an increase in systemic vascular resistance (SVR). The presence of PFO plays an important role in understanding the normal cardiovascular transition physiology and its adverse adaptation. The shunt across the PFO is either left-to-right or bidirectional but never purely right-to-left. A pure right-to-left shunt across the PFO should raise suspicion of underlying CHDs such as total anomalous pulmonary venous drainage or tricuspid atresia. Assessment of the flow across the PFO, especially when it is bidirectional, may indicate persistent pulmonary hypertension of the newborn, and it contributes to monitoring in premature infants and infants with perinatal asphyxia.
In neonates with right heart obstructive lesions, such as tricuspid or pulmonary atresia, and left heart obstructive lesions, such as HLHS, the continued patency of the FO is critical so that an obligatory right-to-left or left-to-right shunt, respectively, across the atrial septum, can take place. In neonates with transposition of the great arteries (TGA), the circulation is parallel (instead of normal in-series circulation), and mixing across the circulations is essential for survival; this is usually provided by the ASD/PFO. With TGA, it is important to assess the atrial level shunt, and a PFO ≤2mm warrants an urgent balloon atrial septostomy to improve survival [9]. The atrial septum in infants with HLHS can be very thick and, if PFO is restrictive, these newborns become critically ill [10]. In this case, instead of a balloon atrial septostomy, a blade septostomy or the creation of a separate defect in the atrial septum by Brockenbrough (needle atrial transeptal) puncture or radiofrequency wire perforation is needed.
4. PFO in Childhood
A PFO is frequently detected during childhood by transthoracic echocardiography (TTE) for the evaluation of a murmur or other medical condition. In the majority of children, TTE with or without contrast saline is sufficient to diagnose a PFO because of the excellent acoustic windows [11]. The most ideal window to visualize the PFO is the subcostal acoustic window. This is because the septum is relatively perpendicular to the transducer and adequately echo-reflective from that position. This reduces the likelihood of a false dropout and a misdiagnosis of a PFO. The confirmation of the PFO is required by documenting evidence of the transseptal flow by color Doppler. Both the subcostal long-axis and short-axis views can be utilized for a confirmatory diagnosis. The apical views often have false dropouts of the acoustic signals due to the parallel orientation of the transducer beam and the atrial septum position. This is not the most ideal location for the evaluation of a PFO. However, the apical-4 chamber view is helpful during the saline contrast “bubble” study in looking for the appearance of acoustic reflective signals (“bubbles”) in the LA and LV (representing right-to-left flow). There is also a need for the standardization of PFO identification and quantification for a saline contrast bubble study, which at present does not exist. In the French PFO- Atrial Septal Aneurysm (ASA) study, a PFO is identified if at least three bubbles appear in the LA. The degree of shunting is defined to be small if three to nine bubbles appear, it is moderate if 10–30 bubbles appear, and large if >30 bubbles appear in the LA [12]. According to PFO in cryptogenic stroke study (PICSS), a PFO is considered to be present if more than one bubble appears in the LA and if >10 appear in the LA, it is considered a large PFO [13]. Recently, it was shown that for a given PFO, the amount of right-to-left contrast shunting is a matter of expiratory pressure during the Valsalva maneuver [14].
Trans-esophageal echocardiogram (TEE) is traditionally considered the gold standard for detection of a PFO in the setting of stroke in adult patients [15][16]. The TEE is necessary for children in order to delineate the anatomy of the interatrial septum during a surgical or interventional cardiac catheterization procedure. In some surgeries, such as tetralogy of Fallot (TOF) repair, a small PFO may be left behind or a punch hole made in the interatrial septum to allow right-to-left shunting and maintain cardiac output in the context of a hypertrophied RV with poor relaxation or potential increased afterload from smallish pulmonary arteries. In these situations, a right-to-left shunt or a bidirectional atrial shunt can be seen on the color Doppler. In other situations, such as a Shone complex with small left-sided structures, there is left-to-right atrial shunting at higher velocities and this evaluation needs to be included in the assessment of transmitral gradients. One major drawback of routine TEE in children is the need for deep sedation or general anesthesia. TEE is a reliable imaging modality to assist with the device closure of a PFO in the cardiac catheterization lab, particularly if a young patient is being anesthetized. In these cases, TEE is useful for determining the precise location (usually the cranial edge of the fossa ovalis), anatomy (slit-like, tunnel-like, aneurysmal or fenestrated), and the direction of shunting, and it can also assist in wire, sheath and device manipulations [17][18][19]. TEE is helpful to evaluate rarely for extension of vegetation in endocarditis, thrombus, or left atrial myxoma. TEE is also a useful adjunct to procedures in which the PFO is traversed or dilated or an atrial shunt is created to allow access to the LA to obtain left-sided hemodynamic data and to perform left-sided interventions to the mitral valve, pulmonary vein stents, etc.
One critical scenario in which a PFO needs to be looked for is in any kind of dilated cardiomyopathy (Figure 3), in which atrial distension is associated with decreased myocardial perfusion. The presence of a PFO or the creation of an atrial level shunt can decrease atrial distension and encourage myocardial recovery in some cases, especially after the placement of an extracorporeal membrane oxygenator (ECMO) [20]. Left ventricular assist device (VAD) implantation is another option for some pediatric patients with refractory heart failure. In these patients, the implantation of a VAD abruptly decreases diastolic filling pressures and can cause right-to-left shunting with profound systemic hypoxemia. The pre-VAD TEE diagnosis of a PFO may be challenged by left atrial distension compressing the defect, which may become more apparent when the LA filling pressures decrease after the VAD placement. A PFO is closed at the time of VAD placement if its presence is detected in time; otherwise, device closure may be subsequently needed [21].
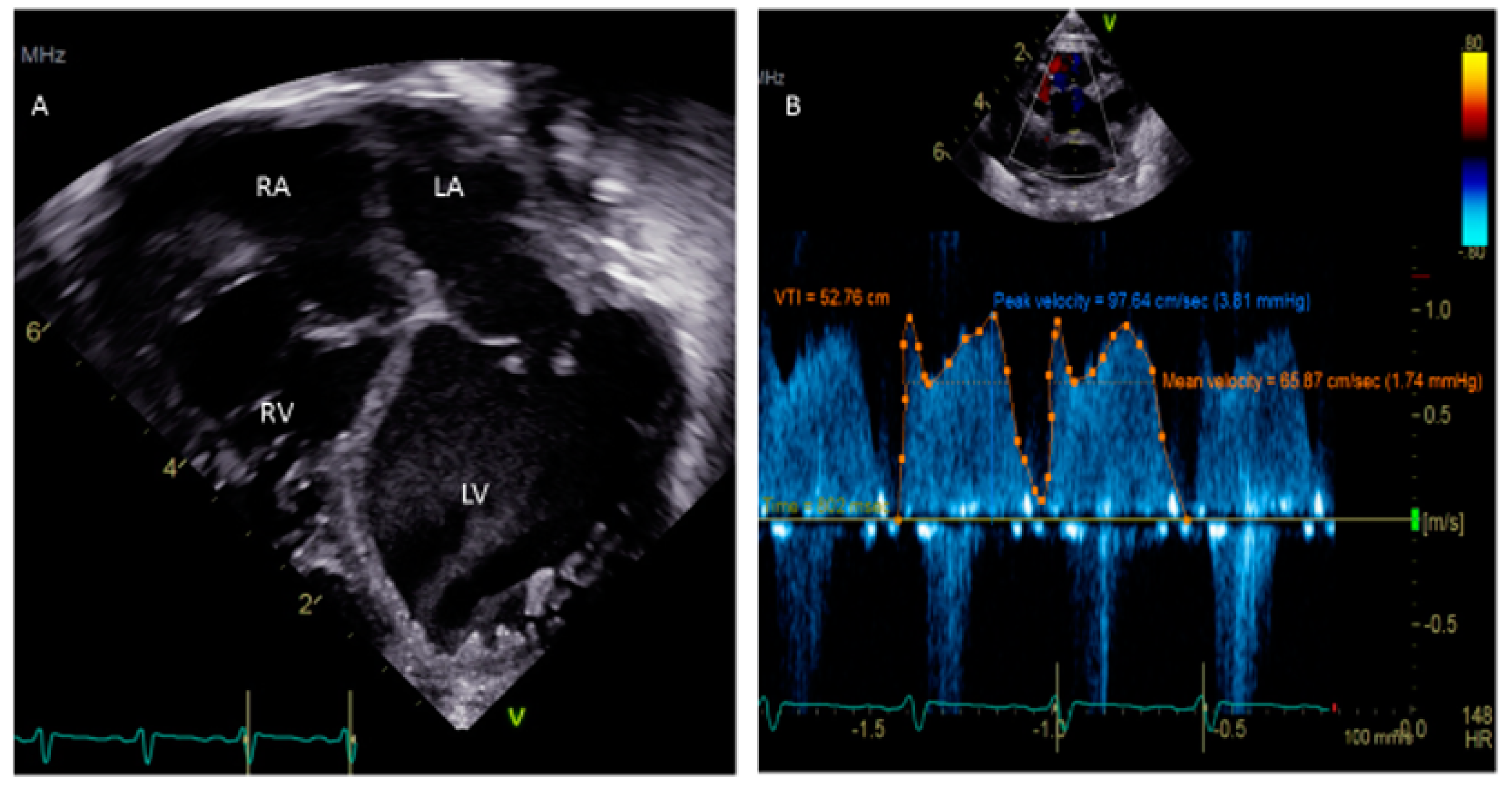
Figure 3. (A) Apical 4-chamber view showing dilated cardiomyopathy in a 5-week-old child. (B) Doppler of PFO shows a peak velocity of 3.81 mm Hg and a mean of 1.74 mm Hg.
5. PFO-Associated Medical Diseases
The left-to-right shunt across the PFO is trivial and not hemodynamically significant, and no treatment is necessary for otherwise asymptomatic children. However, PFOs in the presence of CHDs can be a source of the right-to-left shunt producing paradoxical embolism with resultant cerebrovascular accidents (CVAs)/transient ischemic attacks (TIAs), or brain abscess. In some children, PFO is associated with certain clinical syndromes such as cryptogenic stroke, decompression sickness, migraine, and platypnea-orthodeoxia syndrome.
6. Management of PFO
Currently, there are two approaches to closing a PFO: surgical and transcatheter closures. Surgical closure can be done simultaneously if other surgical interventions are necessary; otherwise, most cardiologists prefer the transcatheter closure of an isolated PFO in special circumstances such as platypnea-orhtodeoxia or when a PFO is causing the exacerbation of systemic hypoxia. There are multiple devices available for transcatheter device closures including Amplatzer™ PFO Occluder (Abbott Inc., Abbott Park, IL) and the Gore Cardioform™Septal Occluder (W. L. Gore & Associates, Flagstaff, AZ) [22]. The FDA currently approves both the devices for transcatheter closure of PFO in the USA. The Amplatzer™ Septal Occluder (Abbott)has been used sometimes in smaller children for PFO closure due to size constraints of the standard Amplatzer PFO occluder device which is available only in 18, 25, and 35 mm diameter sizes. There are also other devices, like Occlutech PFO Occluder (Occlutech), currently approved for use in Europe and other countries. Even though there are adult guidelines with a strong level of evidence supporting the decision-making regarding diagnosis and management of PFO-associated special situations, similar guidelines and supporting evidence are lacking for the pediatric population. In children with PFO-associated special conditions, a personalized approach along with interdisciplinary discussions is necessary prior to any medical or interventional treatment, especially in the background of a lack of robust pediatric data. Future randomized controlled studies with multicenter collaboration are necessary in order to provide the best possible evidence and guide future management of PFO in children. The key areas to focus on would be how to counsel the family of a child with an incidental PFO diagnosis, standardization of diagnosis of incidental isolated PFO, and management of PFO associated special conditions such as stroke, complex migraine, pregnancy and decompression sickness.
References
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT /SCMR/AHA/SOPE 2020 appropriate use of criteria for multimodality imaging during the follow-up care of patients with congenital heart disease. J. Am. Coll. Cardiol. 2020, 75, 657–703.
- Kirk, E.P.; Sunde, M.; Costa, M.W.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and vasculogenesis and cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291.
- Wilmshurst, P.T.; Morrison, W.L.; Walsh, K.P. Comparison of the size of persistent foramen ovale and atrial septal defect in divers with shunt-related decompression illness and the general population. Diving Hyperb. Med. 2015, 45, 89–93.
- Hagen, P.T.; Scholz, D.G.; Edwards, W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin. Proc. 1984, 59, 17–20.
- Allen, H.D.; Shaddy, R.E.; Penny, D.J.; Feltes, T.F.; Cetta, F. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult, 9th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016.
- Moore, K.L.; Persaud, V. The Developing Human: Clinically Oriented Embryology, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 2003.
- Kiserud, T.; Rasmussen, S. Ultrasound assessment of the fetal foramen ovale. Ultrasound Obstet. Gynecol. 2001, 17, 119–124.
- Gu, X.; Zhang, Y.; Han, J.; Liu, X.; He, Y. Isolated premature restriction or closure of foramen ovale in fetuses: Echocardiographic characteristics and outcome. Echocardiography 2018, 35, 1189–1195.
- Songswang, J.; Adatia, I.; Newman, C.; Smallhorn, J.F.; Willaims, W.G.; Freeem, R.M. Mortality in potential arterial switch candidates with transposition of great arteries. J. Am. College Cardiol. 1998, 32, 753–757.
- Jantzen, D.W.; Moon-Grady, A.J.; Morris, S.A.; Armstrong, A.K.; Berg, C.; Gangel, J.; Fifer, C.G.; Frommelt, M.; Gembruch, U.; Herberg, U.; et al. Hypoplastic left heart syndrome with intact or restrictive atrial septum. A report from the International Fetal Cardiac Intervention Registry. Circulation 2017, 136, 1346–1349.
- Hubail, Z.; Lemler, M.; Ramciotti, C.; Moore, J.; Ikemba, C. Diagnosing a patent foramen ovale in children. Is transesophageal echocardiography necessary? Stroke 2011, 42, 98–101.
- Mas, J.L.; Arquizan, C.; Lamy, C.; Zuber, M.; Cabanes, L.; Derumeaux, G.; Coste, J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N. Engl. J. Med. 2001, 345, 1740–1746.
- Homma, S.; Sacco, R.L.; Di Tullio, M.R.; Sciacca, R.R.; Mohr, J.P. Effect of medical treatment in stroke patients with patent foramen ovale. Circulation 2002, 105, 2625–2631.
- Moses, K.L.; Seymour, M.; Beshish, A.; Baker, K.R.; Pegelow, D.F.; Lamers, L.J.; Eldridge, M.W.; Bates, M.L. Inspiratory and expiratory resistance cause right-to-left bubble passage through the foramen ovale. Physiol. Rep. 2018, 6, e13719.
- Lee, R.J.; Bartzokis, T.; Yeoh, T.K.; Frogin, H.R.; Choi, D.; Schnitter, J. Enhanced detection of intracardiac sources of cerebral emboli by transesophageal echocardiography. Stroke 1991, 22, 734–739.
- Chenzbraun, A.; Pinto, F.J.; Schnittger, I. Biplane transesophageal echocardiography in the diagnosis of patent foramen ovale. J. Am. Soc. Echocardiogr. 1993, 6, 417–421.
- Silvestry, F.E. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale from the American Society of Echocardiography for cardiac angiography and interventions. J. Am. Soc. Echocardiogr. 2015, 28, 910–985.
- Hara, H.; Virmani, R.; Ladich, E.; Mackey-Bojack, S.; Titus, J.; Reisman, M.; Gray, W.; Nakamura, M.; Mooney, M.; Poulose, A.; et al. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J. Am. Coll. Cardiol. 2005, 46, 1768–1776.
- Hanley, P.C.; Tajik, A.J.; Hynes, J.K.; Edwards, W.D.; Reeder, G.S.; Hagler, D.J.; Seward, J.B. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: Report of 80 consecutive cases. J. Am. Coll. Cardiol. 1985, 6, 1370–1382.
- Kotani, Y.; Chetan, D.; Rodrigues, W.; Sivarajan, V.B.; Gruenwald, C.; Guerguerian, A.M.; Arsdell, G.S.V.; Honjo, O. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: Current strategy and clinical outcomes. Artif. Organs 2013, 37, 29–36.
- Bacich, D.; Fiorencis, A.; Braggion, G.; Zuin, M.; Rigatelli, G. Patent foramen ovale-related complications in left ventricular assist device patients: A reappraisal for cardiovascular professionals. J. Artif. Organs 2019, 23, 98–104.
- Majunke, N.; Sievert, H. ASD/PFO devices: What is in the pipeline? J. Interv. Cardiol. 2007, 20, 517–523.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
3 times
(View History)
Update Date:
24 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





