Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.; , .; Ye, Q. Common Vaccines against COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/22040 (accessed on 07 February 2026).
Liu Y, , Ye Q. Common Vaccines against COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/22040. Accessed February 07, 2026.
Liu, Ying, , Qing Ye. "Common Vaccines against COVID-19" Encyclopedia, https://encyclopedia.pub/entry/22040 (accessed February 07, 2026).
Liu, Y., , ., & Ye, Q. (2022, April 20). Common Vaccines against COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/22040
Liu, Ying, et al. "Common Vaccines against COVID-19." Encyclopedia. Web. 20 April, 2022.
Copy Citation
To date, multiple COVID-19 vaccines have been granted emergency use authorization, including inactivated vaccines, adenovirus-vectored vaccines, and nucleic acid vaccines. These vaccines have different technical principles, which will necessarily lead to differences in safety and efficacy.
COVID-19
SARS-CoV-2
safety
efficacy
1. Introduction
After the initial outbreak in Wuhan, China, coronavirus disease 2019 (COVID-19) has now spread to more than 200 countries and territories [1]. The worldwide pandemic has imposed a challenge to human health, a significant test of humans facing public health emergencies. Due to ineffective treatments against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccination has become a primary strategy to control the COVID-19 pandemic. In the long term, the establishment of herd immunity by increasing population immunity above a threshold is extremely critical to viral eradication, which not only reduces the spread of the virus from person to person but also indirectly protects unvaccinated, high-risk individuals, including infants, pregnant or breastfeeding women, patients with cancer, immunocompromised people, and so on [2][3]. This can only be done by expanding the vaccinated population to achieve the SARS-CoV-2 herd immunity threshold as fast as possible for specific vaccines. Studies have suggested that the herd immunity threshold for SARS-CoV-2 is approximately 60–70% [4][5]. Therefore, wars against SARS-CoV-2 cannot be comprehensively won without vaccines.
The differences in technical principles and production routes of COVID-19 vaccines will necessarily lead to differences in safety and efficacy [6][7].
2. Concept of Vaccine Design and Mechanism of Action
SARS-CoV-2, the pathogen causing COVID-19, is a positive-stranded enveloped RNA virus containing four main structural proteins, including the spike protein (S), an envelope protein (E), membrane protein (M), and nucleocapsid protein (N) [8][9]. Among them, the S protein, primarily the receptor-binding domain (RBD) on the S1 subunit, mediates cell membrane fusion, facilitates endocytosis, and initiates intracellular signaling related to viral replication by binding to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) [10][11]. Thus, the S protein is the primary target for vaccine design [12]. The role of vaccines is to artificially activate a beneficial immune response by inducing antibody and memory T-like cells. The immune response occurs rapidly upon viral invasion, leading to rapid anamnestic antibody and T cell responses. Circulating antibodies and memory cell recall responses can immediately eliminate viruses and limit virus dissemination [13].
3. Types and Differences of Vaccines
A vaccine from research to marketing ultimately typically requires 5 to 10 years. However, ongoing outbreaks and strong support from drug regulatory authorities greatly accelerate the process of vaccine development. At present, multiple COVID-19 vaccines have been included in the World Health Organization (WHO) emergency use list or have been granted emergency use authorization among different countries, including inactivated vaccines and adenovirus-vectored vaccines, and nucleic acid vaccines [14][15][16][17][18][19]. The vaccines were all given intramuscularly in the deltoid. More details are shown in Table 1.
Table 1. Several granted COVID-19 vaccines and details.
| Vaccine Name | Technology | Developer/Company | Expiration Date | Immunization Protocol | Approved |
|---|---|---|---|---|---|
| CoronaVac | Inactivated vaccine | Sinovac Biotech Ltd. (Beijing, China) |
2–8 °C for 24 months | 2 doses (600SU/0.5 mL/dose), 2–4 weeks apart | WHO 2021.6.1 |
| BBIBP-CorV | Inactivated vaccine | Sinopharm Beijing Institute of Biotechnology (Beijing, China) | 2–8 °C for 24 months | 2 doses (6.5U/0.5 mL/dose), 3–4 weeks apart | WHO 2021.5.7 |
| Convidecia | Adenovirus vector vaccine | Cansino Biologics (Tianjin, China) | 2–8 °C for 12 months | 1 dose (5 × 1010 virus particles/0.5 mL) | China 2021.2. 25 |
| AZD1222 | Adenovirus vector vaccine | AstraZeneca (Cambridge, UK), Oxford University (Oxford, UK) | 2–8 °C for 6 months | 2 dose (5 × 1010 virus particles/0.5 mL), 4–12 weeks apart | WHO 2021.3.1 |
| Ad26.COV2.S | Adenovirus vector vaccine | Johnson & Johnson (New Brunswick, NJ, USA) | 2–8 °C for 3 months | 1 dose (5 × 1010 virus particles/0.5 mL) | WHO 2021.3.17 |
| Sputnik V | Adenovirus vector vaccine | Gamaleya Research Institute (Moscow, Russia) | −18 °C/2–8 °C | 2 dose (1011 viral particles /0.5 mL/dose), 2–3 weeks apart |
Multiple countries without WHO |
| BNT162b2 | mRNA vaccine | Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | Ultralow-temperature freezer for 6 months/−70 ± 10 °C for 10 days/2–8 °C for 5 days | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | WHO 2021.1.14 |
| mRNA-1273 | mRNA vaccine | Moderna (Cambridge, MA, USA) | Between −25 °C and −15 °C for supply/2–8 °C for 30 days | 2 doses (100 μg/0.5 mL/dose), 28 days apart | WHO 2021.2.3 |
| NVX-CoV2373 | Recombinant vaccine | Novavax and the Serum Institute of India (Pune, India) | 2–8 °C for 9 months | 2 doses (55 μg/0.5 mL/dose), 3–4 weeks apart | WHO 2021.12.20 |
Although the above vaccines fulfill the WHO criteria, the fundamental principles and production routes have varied. An illustration of the design principles for different COVID-19 vaccines is presented in Figure 1. Meanwhile, each of them has strengths and weaknesses. Inactivated viral vaccines are a mature technology that has been successfully used in immunization programs for decades, containing inactivated but previously virulent microorganisms that have been destroyed with chemicals, heat, or radiation [20]. In the absence of detailed information about pathogens, inactivated vaccines are the only available vaccine against pandemics [21]. However, inactivated vaccines frequently need to be given in multiple doses, and often a booster dose is necessary to maintain immunity. Examples include BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac). Adenovirus-vectored vaccines are recombinant vaccines formulated by combining the replication-deficient adenovirus vector and the target DNA, such as Convidecia containing replication-defective adenovirus type-5 (Ad5) vectors and the full-length spike gene [22][23]. Therefore, immunized participants can produce S protein and thus, cause a protective immunological response. However, neutralizing antibodies against adenovirus are prevalent in the general population and are likely to weaken the protective efficacy of vaccines [24][25]. Nucleic acid (DNA and RNA) vaccines are novel types of vaccines that work by injecting genetically engineered vectors containing DNA/RNA sequences encoding specific antigens [26]. Nucleic acid vaccines have theoretical advantages over conventional vaccines, which can induce a variety of immune response types at the same time by artificial sequence design [27][28]. Compared with DNA vaccines, mRNA vaccines are safer and more efficient because they avoid the risk of integration with the host cell genome and can produce pure viral protein [29][30][31]. However, mRNA is destabilized and susceptible to degradation, which requires harsh preservation conditions, such as ultralow temperatures. Moreover, none of the mRNA vaccines have been licensed before, and experience in mass production is also scarce. Some mRNA vaccines among the COVID-19 vaccines have received emergency use authorization, such as BNT162b2 and mRNA-1273 developed by Pfizer/BioNTech and Moderna, respectively.
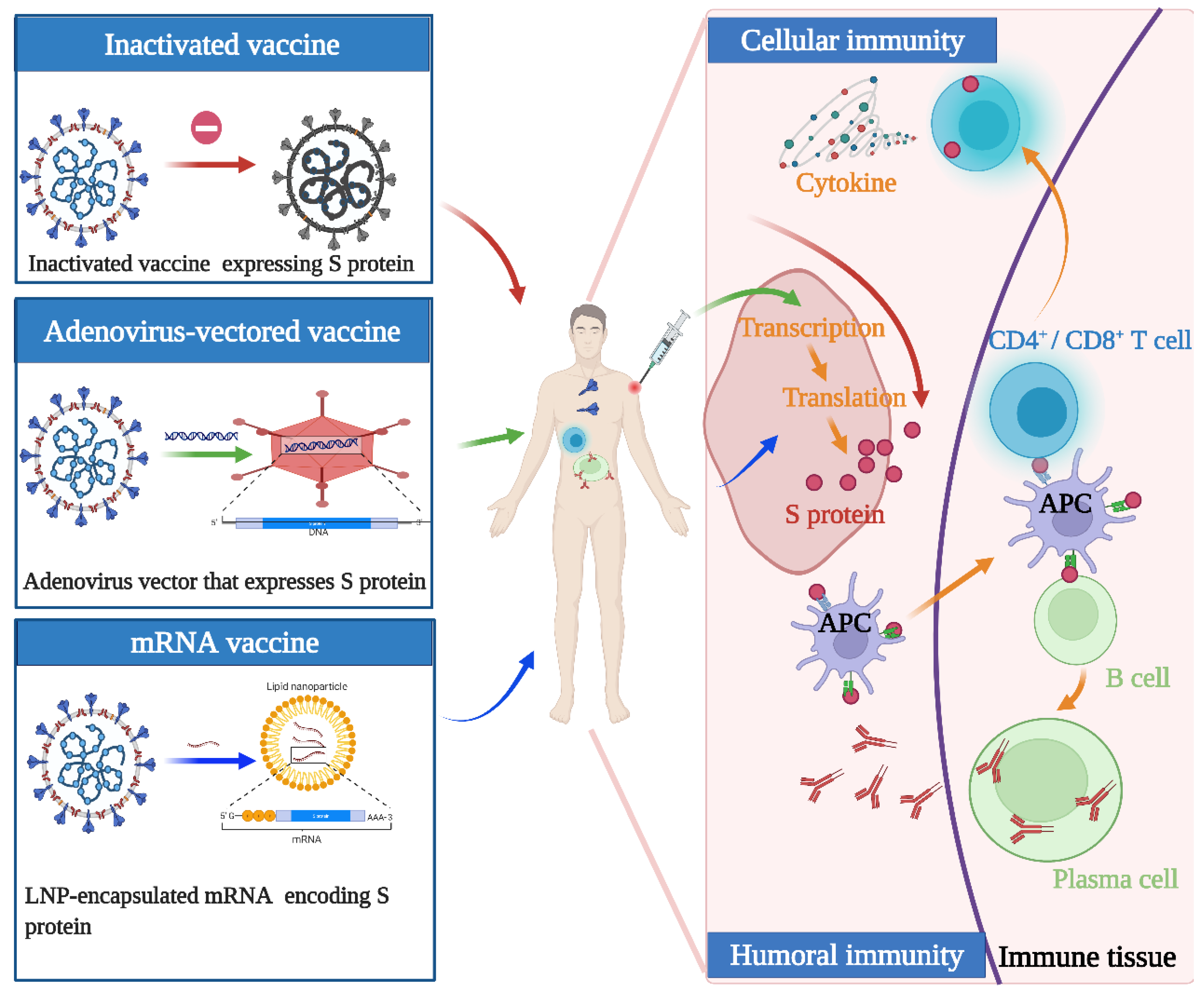
Figure 1. Illustration of the design and operation principles for different COVID-19 vaccines. Inactivated vaccines are inactivated but previously had virulent microorganisms that have been destroyed with chemicals, heat, or radiation. Adenovirus-vectored vaccines are recombinant vaccines formulated by combining the replication-deficient adenovirus vector and the target DNA. mRNA vaccines are a novel type of vaccine that works by injecting genetically engineered vectors containing RNA sequences encoding specific antigens. They function by activating cellular and humoral immunity to varying degrees.
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Okell, L.C.; Verity, R.; Watson, O.J.; Mishra, S.; Walker, P.; Whittaker, C.; Katzourakis, A.; Donnelly, C.A.; Riley, S.; Ghani, A.C.; et al. Have deaths from COVID-19 in Europe plateaued due to herd immunity? Lancet 2020, 395, e110–e111.
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616.
- Angulo, F.J.; Finelli, L.; Swerdlow, D.L. Reopening Society and the Need for Real-Time Assessment of COVID-19 at the Community Level. JAMA 2020, 323, 2247–2248.
- Gomes, M.G.M.; Corder, R.M.; King, J.G.; Langwig, K.E.; Souto-Maior, C.; Carneiro, J.; Gonçalves, G.; Penha-Gonçalves, C.; Ferreira, M.U.; Aguas, R. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv 2020, 540, 111063.
- Holvast, A.; Huckriede, A.; Wilschut, J.; Horst, G.; De Vries, J.J.; Benne, C.A.; Kallenberg, C.G.; Bijl, M. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann. Rheum. Dis. 2006, 65, 913–918.
- Zolla-Pazner, S.; Michael, N.L.; Kim, J.H. A tale of four studies: HIV vaccine immunogenicity and efficacy in clinical trials. Lancet HIV 2021, 8, e449–e452.
- Kanimozhi, G.; Pradhapsingh, B.; Singh Pawar, C.; Khan, H.A.; Alrokayan, S.H.; Prasad, N.R. SARS-CoV-2: Pathogenesis, Molecular Targets and Experimental Models. Front. Pharmacol. 2021, 12, 638334.
- Prates, E.T.; Garvin, M.R.; Pavicic, M.; Jones, P.; Shah, M.; Demerdash, O.; Amos, B.K.; Geiger, A.; Jacobson, D. Potential Pathogenicity Determinants Identified from Structural Proteomics of SARS-CoV and SARS-CoV-2. Mol. Biol. Evol. 2021, 38, 702–715.
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263.
- Zhang, Z.; Zhang, Y.; Liu, K.; Li, Y.; Lu, Q.; Wang, Q.; Zhang, Y.; Wang, L.; Liao, H.; Zheng, A.; et al. The molecular basis for SARS-CoV-2 binding to dog ACE2. Nat. Commun. 2021, 12, 4195.
- Dai, L.; Gao, G.F. WHO Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82.
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287.
- World Health Organization. Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the AZD1222 Vaccine against COVID-19 Developed by Oxford University and AstraZeneca. Available online: https://www.who.int/publications/i/item/background-document-on-the-azd1222-vaccine-against-covid-19-developed-by-oxford-university-and-astrazeneca (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA-1273 Vaccine (Moderna) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-the-mrna-1273-vaccine-(moderna)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Janssen Ad26.COV2. S (COVID-19) Vaccine. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad26.COV2.S-background-2021.1 (accessed on 10 January 2022).
- Li, J.X.; Zhu, F.C. Inactivated SARS-CoV-2 vaccine (BBV152)-induced protection against symptomatic COVID-19. Lancet 2021, 398, 2134–2135.
- Chua, B.Y.; Wong, C.Y.; Mifsud, E.J.; Edenborough, K.M.; Sekiya, T.; Tan, A.C.; Mercuri, F.; Rockman, S.; Chen, W.; Turner, S.J.; et al. Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity. mBio 2015, 6, e01024-15.
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081.
- Roy, C.J.; Ault, A.; Sivasubramani, S.K.; Gorres, J.P.; Wei, C.J.; Andersen, H.; Gall, J.; Roederer, M.; Rao, S.S. Aerosolized adenovirus-vectored vaccine as an alternative vaccine delivery method. Respir. Res. 2011, 12, 153.
- Buchbinder, S.P.; McElrath, M.J.; Dieffenbach, C.; Corey, L. Use of adenovirus type-5 vectored vaccines: A cautionary tale. Lancet 2020, 396, e68–e69.
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9.
- Vogel, F.R.; Sarver, N. Nucleic acid vaccines. Clin. Microbiol. Rev. 1995, 8, 406–410.
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460.
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601.
- Knezevic, I.; Liu, M.A.; Peden, K.; Zhou, T.; Kang, H.N. Development of mRNA Vaccines: Scientific and Regulatory Issues. Vaccines 2021, 9, 81.
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147.
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
900
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




