Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
To date, multiple COVID-19 vaccines have been granted emergency use authorization, including inactivated vaccines, adenovirus-vectored vaccines, and nucleic acid vaccines. These vaccines have different technical principles, which will necessarily lead to differences in safety and efficacy.
- COVID-19
- SARS-CoV-2
- safety
- efficacy
1. Introduction
After the initial outbreak in Wuhan, China, coronavirus disease 2019 (COVID-19) has now spread to more than 200 countries and territories [1]. The worldwide pandemic has imposed a challenge to human health, a significant test of humans facing public health emergencies. Due to ineffective treatments against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccination has become a primary strategy to control the COVID-19 pandemic. In the long term, the establishment of herd immunity by increasing population immunity above a threshold is extremely critical to viral eradication, which not only reduces the spread of the virus from person to person but also indirectly protects unvaccinated, high-risk individuals, including infants, pregnant or breastfeeding women, patients with cancer, immunocompromised people, and so on [2][3]. This can only be done by expanding the vaccinated population to achieve the SARS-CoV-2 herd immunity threshold as fast as possible for specific vaccines. Studies have suggested that the herd immunity threshold for SARS-CoV-2 is approximately 60–70% [4][5]. Therefore, wars against SARS-CoV-2 cannot be comprehensively won without vaccines.
The differences in technical principles and production routes of COVID-19 vaccines will necessarily lead to differences in safety and efficacy [6][7].
2. Concept of Vaccine Design and Mechanism of Action
SARS-CoV-2, the pathogen causing COVID-19, is a positive-stranded enveloped RNA virus containing four main structural proteins, including the spike protein (S), an envelope protein (E), membrane protein (M), and nucleocapsid protein (N) [8][9]. Among them, the S protein, primarily the receptor-binding domain (RBD) on the S1 subunit, mediates cell membrane fusion, facilitates endocytosis, and initiates intracellular signaling related to viral replication by binding to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) [10][11]. Thus, the S protein is the primary target for vaccine design [12]. The role of vaccines is to artificially activate a beneficial immune response by inducing antibody and memory T-like cells. The immune response occurs rapidly upon viral invasion, leading to rapid anamnestic antibody and T cell responses. Circulating antibodies and memory cell recall responses can immediately eliminate viruses and limit virus dissemination [13].
3. Types and Differences of Vaccines
A vaccine from research to marketing ultimately typically requires 5 to 10 years. However, ongoing outbreaks and strong support from drug regulatory authorities greatly accelerate the process of vaccine development. At present, multiple COVID-19 vaccines have been included in the World Health Organization (WHO) emergency use list or have been granted emergency use authorization among different countries, including inactivated vaccines and adenovirus-vectored vaccines, and nucleic acid vaccines [14][15][16][17][18][19]. The vaccines were all given intramuscularly in the deltoid. More details are shown in Table 1.
Table 1. Several granted COVID-19 vaccines and details.
| Vaccine Name | Technology | Developer/Company | Expiration Date | Immunization Protocol | Approved |
|---|---|---|---|---|---|
| CoronaVac | Inactivated vaccine | Sinovac Biotech Ltd. (Beijing, China) |
2–8 °C for 24 months | 2 doses (600SU/0.5 mL/dose), 2–4 weeks apart | WHO 2021.6.1 |
| BBIBP-CorV | Inactivated vaccine | Sinopharm Beijing Institute of Biotechnology (Beijing, China) | 2–8 °C for 24 months | 2 doses (6.5U/0.5 mL/dose), 3–4 weeks apart | WHO 2021.5.7 |
| Convidecia | Adenovirus vector vaccine | Cansino Biologics (Tianjin, China) | 2–8 °C for 12 months | 1 dose (5 × 1010 virus particles/0.5 mL) | China 2021.2. 25 |
| AZD1222 | Adenovirus vector vaccine | AstraZeneca (Cambridge, UK), Oxford University (Oxford, UK) | 2–8 °C for 6 months | 2 dose (5 × 1010 virus particles/0.5 mL), 4–12 weeks apart | WHO 2021.3.1 |
| Ad26.COV2.S | Adenovirus vector vaccine | Johnson & Johnson (New Brunswick, NJ, USA) | 2–8 °C for 3 months | 1 dose (5 × 1010 virus particles/0.5 mL) | WHO 2021.3.17 |
| Sputnik V | Adenovirus vector vaccine | Gamaleya Research Institute (Moscow, Russia) | −18 °C/2–8 °C | 2 dose (1011 viral particles /0.5 mL/dose), 2–3 weeks apart |
Multiple countries without WHO |
| BNT162b2 | mRNA vaccine | Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | Ultralow-temperature freezer for 6 months/−70 ± 10 °C for 10 days/2–8 °C for 5 days | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | WHO 2021.1.14 |
| mRNA-1273 | mRNA vaccine | Moderna (Cambridge, MA, USA) | Between −25 °C and −15 °C for supply/2–8 °C for 30 days | 2 doses (100 μg/0.5 mL/dose), 28 days apart | WHO 2021.2.3 |
| NVX-CoV2373 | Recombinant vaccine | Novavax and the Serum Institute of India (Pune, India) | 2–8 °C for 9 months | 2 doses (55 μg/0.5 mL/dose), 3–4 weeks apart | WHO 2021.12.20 |
Although the above vaccines fulfill the WHO criteria, the fundamental principles and production routes have varied. An illustration of the design principles for different COVID-19 vaccines is presented in Figure 1. Meanwhile, each of them has strengths and weaknesses. Inactivated viral vaccines are a mature technology that has been successfully used in immunization programs for decades, containing inactivated but previously virulent microorganisms that have been destroyed with chemicals, heat, or radiation [20]. In the absence of detailed information about pathogens, inactivated vaccines are the only available vaccine against pandemics [21]. However, inactivated vaccines frequently need to be given in multiple doses, and often a booster dose is necessary to maintain immunity. Examples include BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac). Adenovirus-vectored vaccines are recombinant vaccines formulated by combining the replication-deficient adenovirus vector and the target DNA, such as Convidecia containing replication-defective adenovirus type-5 (Ad5) vectors and the full-length spike gene [22][23]. Therefore, immunized participants can produce S protein and thus, cause a protective immunological response. However, neutralizing antibodies against adenovirus are prevalent in the general population and are likely to weaken the protective efficacy of vaccines [24][25]. Nucleic acid (DNA and RNA) vaccines are novel types of vaccines that work by injecting genetically engineered vectors containing DNA/RNA sequences encoding specific antigens [26]. Nucleic acid vaccines have theoretical advantages over conventional vaccines, which can induce a variety of immune response types at the same time by artificial sequence design [27][28]. Compared with DNA vaccines, mRNA vaccines are safer and more efficient because they avoid the risk of integration with the host cell genome and can produce pure viral protein [29][30][31]. However, mRNA is destabilized and susceptible to degradation, which requires harsh preservation conditions, such as ultralow temperatures. Moreover, none of the mRNA vaccines have been licensed before, and experience in mass production is also scarce. Some mRNA vaccines among the COVID-19 vaccines have received emergency use authorization, such as BNT162b2 and mRNA-1273 developed by Pfizer/BioNTech and Moderna, respectively.
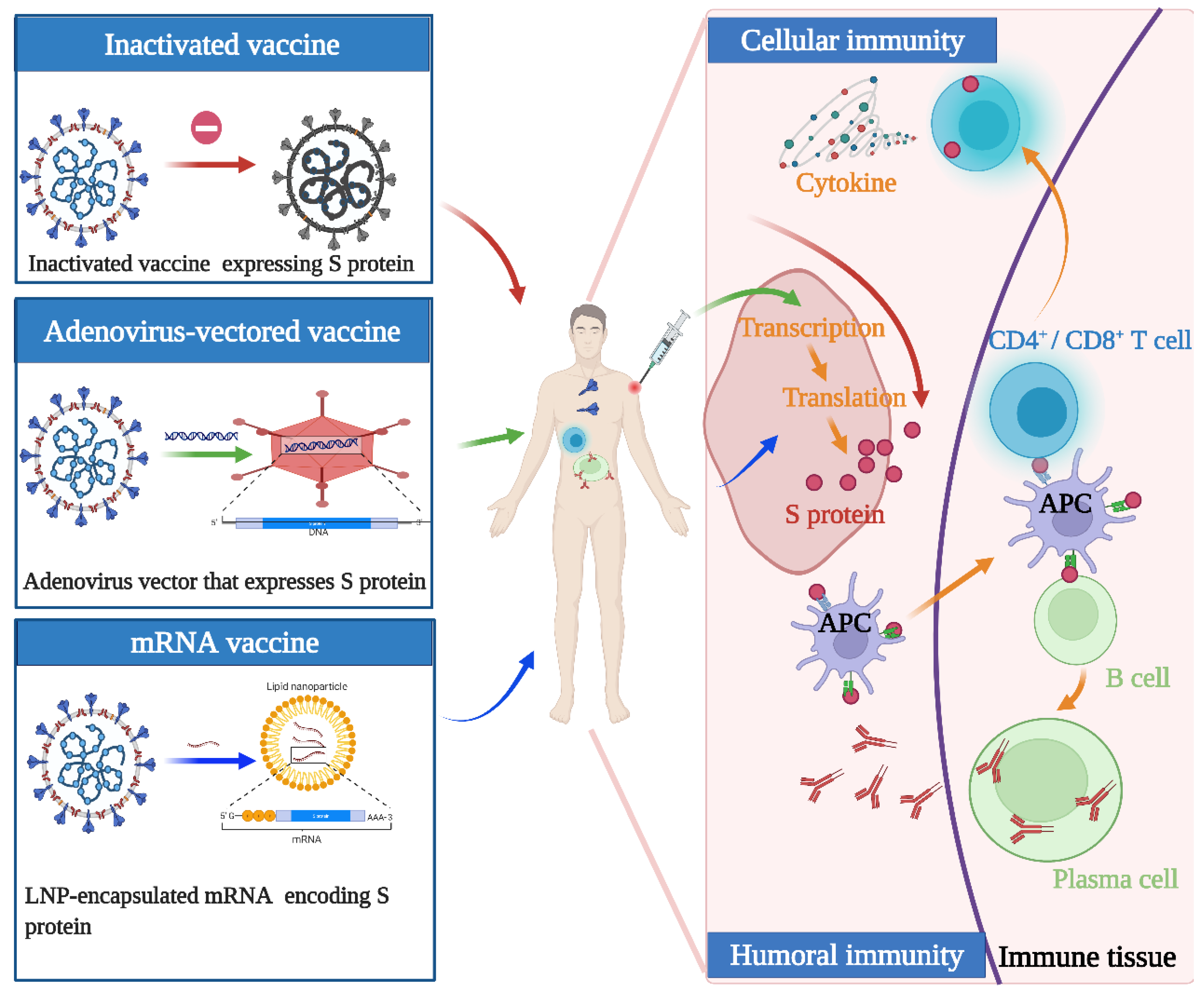
Figure 1. Illustration of the design and operation principles for different COVID-19 vaccines. Inactivated vaccines are inactivated but previously had virulent microorganisms that have been destroyed with chemicals, heat, or radiation. Adenovirus-vectored vaccines are recombinant vaccines formulated by combining the replication-deficient adenovirus vector and the target DNA. mRNA vaccines are a novel type of vaccine that works by injecting genetically engineered vectors containing RNA sequences encoding specific antigens. They function by activating cellular and humoral immunity to varying degrees.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10040513
This entry is offline, you can click here to edit this entry!
