
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto Bartolomé | -- | 2393 | 2022-04-20 12:45:11 | | | |
| 2 | Peter Tang | Meta information modification | 2393 | 2022-04-21 03:15:03 | | |
Video Upload Options
β-cells are insulin-producing cells in the pancreas that maintain euglycemic conditions. Pancreatic β-cell maturity and function are regulated by a variety of transcription factors that enable the adequate expression of the cellular machinery involved in nutrient sensing and commensurate insulin secretion. One of the key factors in this regulation is MAF bZIP transcription factor A (MafA). MafA expression is decreased in type 2 diabetes, contributing to β-cell dysfunction and disease progression. The molecular biology underlying MafA is complex, with numerous transcriptional and post-translational regulatory nodes.
1. Introduction
2. MafA Regulates β-Cell Function
3. MafA Target Genes
|
Gene Symbol |
Gene Name |
Gene Function |
Reference |
|---|---|---|---|
|
Cacng4 * |
Voltage-dependent calcium channel gamma-4 subunit |
Enhances L-type Ca2+-mediated Ca2+ entry into β-cell |
[42] |
|
Chrnb4 * |
Cholinergic receptor nicotinic beta 4 |
Subunit of the nicotinic acetylcholine receptor |
[43] |
|
Cox6a2 * |
Cytochrome C oxidase subunit 6A2 |
One out of 13 subunits of cytochrome C oxidase complex (Complex IV), the last enzyme in the electron transport chain |
[44] |
|
G6pc2 * |
Glucose-6-phosphate catalytic subunit related protein |
Islet-specific enzyme that hydrolyzes glucose-6-phosphate, limits basal insulin secretion |
[45] |
|
Gck |
Glucokinase |
Phosphorylates glucose to glucose-6-phosphate in pancreatic islets and hepatocytes. Considered the β-cell glucose sensor |
[46] |
|
Glp1r |
Glucagon-like peptide 1 receptor |
Receptor for Glucagon-like peptide 1 (Glp1), a stimulator of insulin secretion |
[46] |
|
Ins1 * |
Insulin I |
One of two insulin genes in mouse, on chromosome 19 |
[11] |
|
Ins2 * |
Insulin II |
One of two insulin genes in mouse, on chromosome 7 |
[11] |
|
Maob * |
Monoamine oxidase B |
Metabolizes monoamine neurotransmitters, specifically benzylamine, dopamine and phenylethylamine |
[47] |
|
Neurod1 |
Neurogenic differentiation 1 |
Transactivator of genes important for β-cell maturation and function, including insulin |
[46] |
|
Nkx6.1 |
NK6 homeobox1 |
TF involved in β-cell development and regulation of genes involved in mature β-cell function |
|
|
Pcsk1 |
Proprotein convertase subtilisin/kexin type 1 |
Proprotein convertase, which processes proinsulin in β-cells |
[46] |
|
Pcx |
Pyruvate carboxylase |
Catalyzes the conversion of pyruvate to oxaloacetate |
[46] |
|
Pdx1 * |
Pancreatic and duodenal homeobox 1 |
TF important pancreas development and for mature β-cell function |
|
|
PPP1R1A |
Protein phosphatase 1, regulatory inhibitor subunit 1A |
Regulates cAMP/PKA signaling pathway Promotes Glp1-induced GSIS |
[50] |
|
Prlr |
Prolactin Receptor |
Involved in increasing β-cell mass during pregnancy |
[51] |
|
Slc2a2 * |
Solute Carrier Family 2 Member 2 |
Glucose transporter 2, transmembrane glucose transporter with a high Km for glucose |
|
|
Slc80a8 |
Solute carrier family 30 member 8 |
Zinc transporter on insulin granules in β-cells |
4. Regulation of MafA Transcription
|
Protein Symbol |
Protein Name |
Mafa Transcription Mechanism |
Reference |
|---|---|---|---|
|
Pax6 |
Paired box protein Pax-6 |
Binds R1, R3 and R6 of the Mafa promoter |
[55] |
|
Nkx6.1 |
NK6 homeobox 1 |
Binds R3 of the Mafa promoter |
[55] |
|
Nkx2.2 |
NK2 homeobox 2 |
Binds R3 of the Mafa promoter |
[54] |
|
Pdx1 |
Pancreatic and duodenal homeobox 1 |
Binds R3 and R6 of the Mafa promoter |
|
|
Hnf1a |
Hepatocyte nuclear factor 1-alpha |
Binds R3 of the Mafa promoter |
[53] |
|
Foxa2 |
Forkhead box A2 |
Binds R3 of the Mafa promoter |
[54] |
|
Isl1 |
Insulin gene enhancer protein ISL-1 |
Binds R3 of the Mafa promoter |
[59] |
|
Neurod1 |
Neurogenic differentiation 1 |
Binds R3 of the Mafa promoter |
[54] |
|
Pax4 |
Paired box protein Pax-4 |
Negative regulator of Mafa, potentially by interfering other factors from binding R3 of the Mafa promoter |
[57] |
|
Mafb |
Transcription factor MafB |
Binds R3 of the Mafa promoter |
|
|
Onecut1 |
One cut domain, family member 1 |
Prevents Foxa2 from binding to the Mafa promoter |
[58] |
|
Foxo1 |
Forkhead box O1 |
Binds to the forkhead element of the Mafa promoter |
[32] |
|
Thra |
Thyroid hormone receptor alpha |
Binds to Thyroid hormone response element (TRE), which are located from −1927 to −1946 and from +647 to +659 (named Site 2 and Site 3, respectively) |
[61] |
|
CREB |
cAMP responsive element binding protein |
Constitutively binds to the cAMP response element (CRE), spanning from −1342 to −1346, of the Mafa promoter |
[62] |
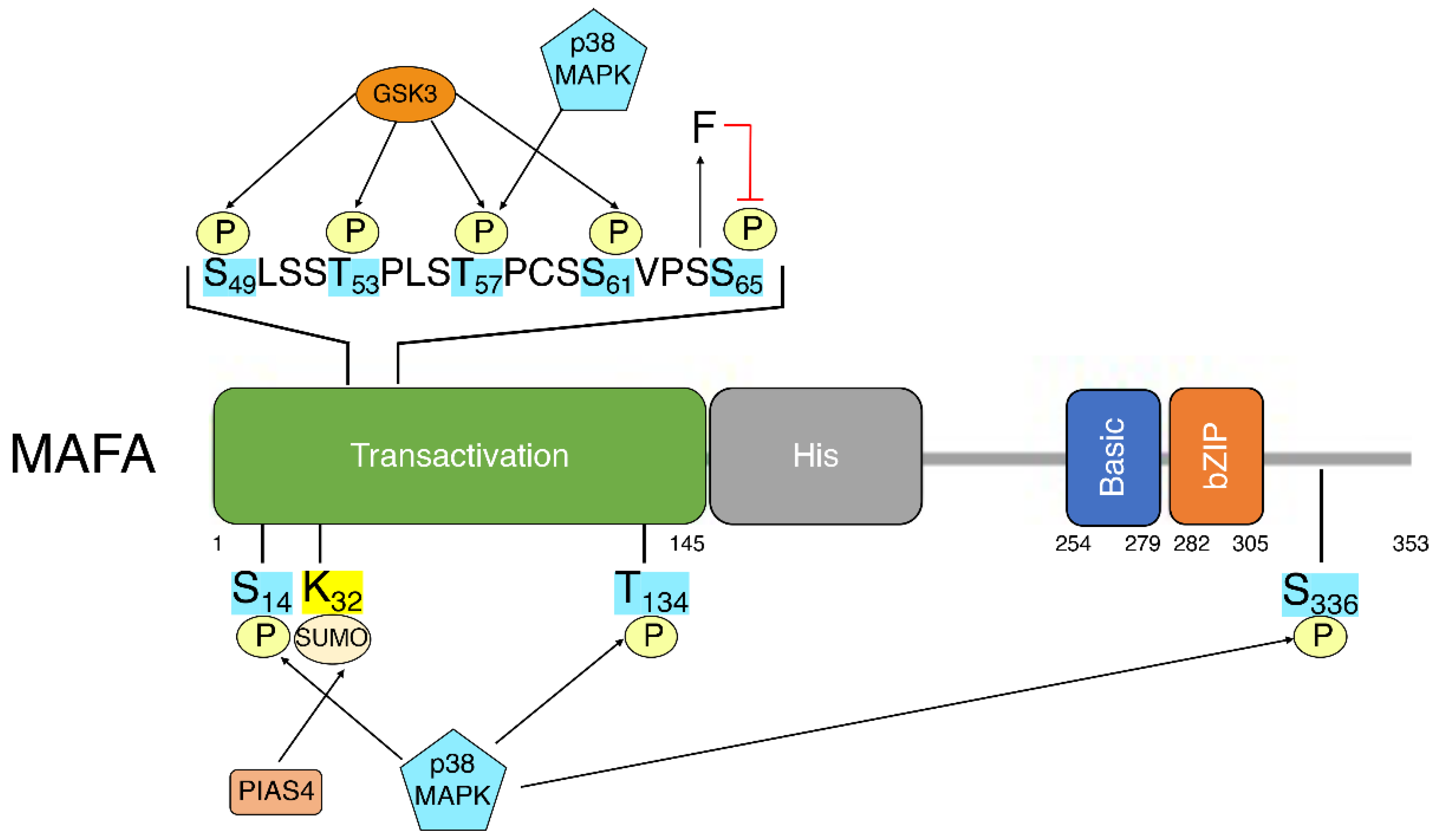
5. MafA Post-Translational Modifications
References
- Nishizawa, M.; Kataoka, K.; Goto, N.; Fujiwara, K.T.; Kawai, S. V-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc. Natl. Acad. Sci. USA 1989, 86, 7711–7715.
- Reza, H.M.; Yasuda, K. Roles of Maf family proteins in lens development. Dev. Dyn. 2004, 229, 440–448.
- Zhang, C.; Guo, Z.M. Multiple functions of Maf in the regulation of cellular development and differentiation. Diabetes Metab. Res. Rev. 2015, 31, 773–778.
- Artner, I.; Le Lay, J.; Hang, Y.; Elghazi, L.; Schisler, J.C.; Henderson, E.; Sosa-Pineda, B.; Stein, R. MafB: An activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes 2006, 55, 297–304.
- Eychene, A.; Rocques, N.; Pouponnot, C. A new MAFia in cancer. Nat. Rev. Cancer 2008, 8, 683–693.
- Olbrot, M.; Rud, J.; Moss, L.G.; Sharma, A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. USA 2002, 99, 6737–6742.
- Kataoka, K.; Han, S.I.; Shioda, S.; Hirai, M.; Nishizawa, M.; Handa, H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J. Biol. Chem. 2002, 277, 49903–49910.
- Matsuoka, T.A.; Zhao, L.; Artner, I.; Jarrett, H.W.; Friedman, D.; Means, A.; Stein, R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 2003, 23, 6049–6062.
- Matsuoka, T.A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, T.; Yamamoto, K.; Kato, K.; Shimomura, I.; Stein, R.; Matsuhisa, M. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes 2010, 59, 1709–1720.
- Guo, S.; Dai, C.; Guo, M.; Taylor, B.; Harmon, J.S.; Sander, M.; Robertson, R.P.; Powers, A.C.; Stein, R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013, 123, 3305–3316.
- Zhang, C.; Moriguchi, T.; Kajihara, M.; Esaki, R.; Harada, A.; Shimohata, H.; Oishi, H.; Hamada, M.; Morito, N.; Hasegawa, K.; et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 2005, 25, 4969–4976.
- Nishimura, W.; Takahashi, S.; Yasuda, K. MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia 2015, 58, 566–574.
- Aramata, S.; Han, S.I.; Yasuda, K.; Kataoka, K. Synergistic activation of the insulin gene promoter by the beta-cell enriched transcription factors MafA, Beta2, and Pdx1. Biochim. Biophys. Acta 2005, 1730, 41–46.
- Zhao, L.; Guo, M.; Matsuoka, T.A.; Hagman, D.K.; Parazzoli, S.D.; Poitout, V.; Stein, R. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J. Biol. Chem. 2005, 280, 11887–11894.
- Artner, I.; Hang, Y.; Guo, M.; Gu, G.; Stein, R. MafA is a dedicated activator of the insulin gene in vivo. J. Endocrinol. 2008, 198, 271–279.
- Matsuoka, T.A.; Artner, I.; Henderson, E.; Means, A.; Sander, M.; Stein, R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. USA 2004, 101, 2930–2933.
- Matsuoka, T.A.; Kaneto, H.; Stein, R.; Miyatsuka, T.; Kawamori, D.; Henderson, E.; Kojima, I.; Matsuhisa, M.; Hori, M.; Yamasaki, Y. MafA regulates expression of genes important to islet beta-cell function. Mol. Endocrinol. 2007, 21, 2764–2774.
- Aguayo-Mazzucato, C.; Koh, A.; El Khattabi, I.; Li, W.C.; Toschi, E.; Jermendy, A.; Juhl, K.; Mao, K.; Weir, G.C.; Sharma, A.; et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 2011, 54, 583–593.
- Artner, I.; Hang, Y.; Mazur, M.; Yamamoto, T.; Guo, M.; Lindner, J.; Magnuson, M.A.; Stein, R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 2010, 59, 2530–2539.
- He, K.H.; Juhl, K.; Karadimos, M.; El Khattabi, I.; Fitzpatrick, C.; Bonner-Weir, S.; Sharma, A. Differentiation of pancreatic endocrine progenitors reversibly blocked by premature induction of MafA. Dev. Biol. 2014, 385, 2–12.
- Nishimura, W.; Bonner-Weir, S.; Sharma, A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev. Biol. 2009, 333, 108–120.
- Hang, Y.; Yamamoto, T.; Benninger, R.K.; Brissova, M.; Guo, M.; Bush, W.; Piston, D.W.; Powers, A.C.; Magnuson, M.; Thurmond, D.C.; et al. The MafA transcription factor becomes essential to islet beta-cells soon after birth. Diabetes 2014, 63, 1994–2005.
- Katoh, M.C.; Jung, Y.; Ugboma, C.M.; Shimbo, M.; Kuno, A.; Basha, W.A.; Kudo, T.; Oishi, H.; Takahashi, S. MafB Is Critical for Glucagon Production and Secretion in Mouse Pancreatic alpha Cells In Vivo. Mol. Cell. Biol. 2018, 38, e00504-17.
- Cyphert, H.A.; Walker, E.M.; Hang, Y.; Dhawan, S.; Haliyur, R.; Bonatakis, L.; Avrahami, D.; Brissova, M.; Kaestner, K.H.; Bhushan, A.; et al. Examining How the MAFB Transcription Factor Affects Islet beta-Cell Function Postnatally. Diabetes 2019, 68, 337–348.
- Conrad, E.; Dai, C.; Spaeth, J.; Guo, M.; Cyphert, H.A.; Scoville, D.; Carroll, J.; Yu, W.M.; Goodrich, L.V.; Harlan, D.M.; et al. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E91–E102.
- Xiafukaiti, G.; Maimaiti, S.; Ogata, K.; Kuno, A.; Kudo, T.; Shawki, H.H.; Oishi, H.; Takahashi, S. MafB Is Important for Pancreatic beta-Cell Maintenance under a MafA-Deficient Condition. Mol. Cell. Biol. 2019, 39, e00080-19.
- Bonnavion, R.; Jaafar, R.; Kerr-Conte, J.; Assade, F.; van Stralen, E.; Leteurtre, E.; Pouponnot, C.; Gargani, S.; Pattou, F.; Bertolino, P.; et al. Both PAX4 and MAFA are expressed in a substantial proportion of normal human pancreatic alpha cells and deregulated in patients with type 2 diabetes. PLoS ONE 2013, 8, e72194.
- Butler, A.E.; Robertson, R.P.; Hernandez, R.; Matveyenko, A.V.; Gurlo, T.; Butler, P.C. Beta cell nuclear musculoaponeurotic fibrosarcoma oncogene family A (MafA) is deficient in type 2 diabetes. Diabetologia 2012, 55, 2985–2988.
- Cinti, F.; Bouchi, R.; Kim-Muller, J.Y.; Ohmura, Y.; Sandoval, P.R.; Masini, M.; Marselli, L.; Suleiman, M.; Ratner, L.E.; Marchetti, P.; et al. Evidence of beta-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1044–1054.
- Matsuoka, T.A.; Kaneto, H.; Kawashima, S.; Miyatsuka, T.; Tochino, Y.; Yoshikawa, A.; Imagawa, A.; Miyazaki, J.; Gannon, M.; Stein, R.; et al. Preserving Mafa expression in diabetic islet beta-cells improves glycemic control in vivo. J. Biol. Chem. 2015, 290, 7647–7657.
- Son, J.; Ding, H.; Farb, T.B.; Efanov, A.M.; Sun, J.; Gore, J.L.; Syed, S.K.; Lei, Z.; Wang, Q.; Accili, D.; et al. BACH2 inhibition reverses beta cell failure in type 2 diabetes models. J. Clin. Investig. 2021, 131, e153876.
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005, 2, 153–163.
- Shrestha, S.; Saunders, D.C.; Walker, J.T.; Camunas-Soler, J.; Dai, X.Q.; Haliyur, R.; Aramandla, R.; Poffenberger, G.; Prasad, N.; Bottino, R.; et al. Combinatorial transcription factor profiles predict mature and functional human islet alpha and beta cells. JCI Insight 2021, 6, e151621.
- Benkhelifa, S.; Provot, S.; Lecoq, O.; Pouponnot, C.; Calothy, G.; Felder-Schmittbuhl, M.P. mafA, a novel member of the maf proto-oncogene family, displays developmental regulation and mitogenic capacity in avian neuroretina cells. Oncogene 1998, 17, 247–254.
- Camunas-Soler, J.; Dai, X.Q.; Hang, Y.; Bautista, A.; Lyon, J.; Suzuki, K.; Kim, S.K.; Quake, S.R.; MacDonald, P.E. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metab. 2020, 31, 1017–1031.e1014.
- Lu, X.; Guanga, G.P.; Wan, C.; Rose, R.B. A novel DNA binding mechanism for maf basic region-leucine zipper factors inferred from a MafA-DNA complex structure and binding specificities. Biochemistry 2012, 51, 9706–9717.
- Kataoka, K.; Noda, M.; Nishizawa, M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol. Cell. Biol. 1994, 14, 700–712.
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Kinjo, T.; Saitoh, I.; Watanabe, M. Mutations in the C1 element of the insulin promoter lead to diabetic phenotypes in homozygous mice. Commun. Biol. 2020, 3, 309.
- Garin, I.; Edghill, E.L.; Akerman, I.; Rubio-Cabezas, O.; Rica, I.; Locke, J.M.; Maestro, M.A.; Alshaikh, A.; Bundak, R.; del Castillo, G.; et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3105–3110.
- Akerman, I.; Maestro, M.A.; De Franco, E.; Grau, V.; Flanagan, S.; Garcia-Hurtado, J.; Mittler, G.; Ravassard, P.; Piemonti, L.; Ellard, S.; et al. Neonatal diabetes mutations disrupt a chromatin pioneering function that activates the human insulin gene. Cell Rep. 2021, 35, 108981.
- ZeRuth, G.T.; Takeda, Y.; Jetten, A.M. The Kruppel-like protein Gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol. Endocrinol. 2013, 27, 1692–1705.
- Luan, C.; Ye, Y.; Singh, T.; Barghouth, M.; Eliasson, L.; Artner, I.; Zhang, E.; Renstrom, E. The calcium channel subunit gamma-4 is regulated by MafA and necessary for pancreatic beta-cell specification. Commun. Biol. 2019, 2, 106.
- Ganic, E.; Singh, T.; Luan, C.; Fadista, J.; Johansson, J.K.; Cyphert, H.A.; Bennet, H.; Storm, P.; Prost, G.; Ahlenius, H.; et al. MafA-Controlled Nicotinic Receptor Expression Is Essential for Insulin Secretion and Is Impaired in Patients with Type 2 Diabetes. Cell Rep. 2016, 14, 1991–2002.
- Nagai, Y.; Matsuoka, T.A.; Shimo, N.; Miyatsuka, T.; Miyazaki, S.; Tashiro, F.; Miyazaki, J.I.; Katakami, N.; Shimomura, I. Glucotoxicity-induced suppression of Cox6a2 expression provokes beta-cell dysfunction via augmented ROS production. Biochem. Biophys. Res. Commun. 2021, 556, 134–141.
- Martin, C.C.; Flemming, B.P.; Wang, Y.; Oeser, J.K.; O’Brien, R.M. Foxa2 and MafA regulate islet-specific glucose-6-phosphatase catalytic subunit-related protein gene expression. J. Mol. Endocrinol. 2008, 41, 315–328.
- Wang, H.; Brun, T.; Kataoka, K.; Sharma, A.J.; Wollheim, C.B. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 2007, 50, 348–358.
- Ganic, E.; Johansson, J.K.; Bennet, H.; Fex, M.; Artner, I. Islet-specific monoamine oxidase A and B expression depends on MafA transcriptional activity and is compromised in type 2 diabetes. Biochem. Biophys. Res. Commun. 2015, 468, 629–635.
- Aigha, I.I.; Abdelalim, E.M. NKX6.1 transcription factor: A crucial regulator of pancreatic beta cell development, identity, and proliferation. Stem Cell Res. Ther. 2020, 11, 459.
- Vanhoose, A.M.; Samaras, S.; Artner, I.; Henderson, E.; Hang, Y.; Stein, R. MafA and MafB regulate Pdx1 transcription through the Area II control region in pancreatic beta cells. J. Biol. Chem. 2008, 283, 22612–22619.
- Cataldo, L.R.; Vishnu, N.; Singh, T.; Bertonnier-Brouty, L.; Bsharat, S.; Luan, C.; Renstrom, E.; Prasad, R.B.; Fex, M.; Mulder, H.; et al. The MafA-target gene PPP1R1A regulates GLP1R-mediated amplification of glucose-stimulated insulin secretion in beta-cells. Metabolism 2021, 118, 154734.
- Eto, K.; Nishimura, W.; Oishi, H.; Udagawa, H.; Kawaguchi, M.; Hiramoto, M.; Fujiwara, T.; Takahashi, S.; Yasuda, K. MafA is required for postnatal proliferation of pancreatic beta-cells. PLoS ONE 2014, 9, e104184.
- Ono, Y.; Kataoka, K. MafA, NeuroD1, and HNF1beta synergistically activate the Slc2a2 (Glut2) gene in beta-cells. J. Mol. Endocrinol. 2021, 67, 71–82.
- Hunter, C.S.; Maestro, M.A.; Raum, J.C.; Guo, M.; Thompson, F.H., 3rd; Ferrer, J.; Stein, R. Hnf1alpha (MODY3) regulates beta-cell-enriched MafA transcription factor expression. Mol. Endocrinol. 2011, 25, 339–347.
- Raum, J.C.; Gerrish, K.; Artner, I.; Henderson, E.; Guo, M.; Sussel, L.; Schisler, J.C.; Newgard, C.B.; Stein, R. FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol. Cell. Biol. 2006, 26, 5735–5743.
- Raum, J.C.; Hunter, C.S.; Artner, I.; Henderson, E.; Guo, M.; Elghazi, L.; Sosa-Pineda, B.; Ogihara, T.; Mirmira, R.G.; Sussel, L.; et al. Islet beta-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol. Cell. Biol. 2010, 30, 4234–4244.
- Romer, A.I.; Singer, R.A.; Sui, L.; Egli, D.; Sussel, L. Murine Perinatal beta-Cell Proliferation and the Differentiation of Human Stem Cell-Derived Insulin-Expressing Cells Require NEUROD1. Diabetes 2019, 68, 2259–2271.
- Hu He, K.H.; Lorenzo, P.I.; Brun, T.; Jimenez Moreno, C.M.; Aeberhard, D.; Vallejo Ortega, J.; Cornu, M.; Thorel, F.; Gjinovci, A.; Thorens, B.; et al. In vivo conditional Pax4 overexpression in mature islet beta-cells prevents stress-induced hyperglycemia in mice. Diabetes 2011, 60, 1705–1715.
- Yamamoto, K.; Matsuoka, T.A.; Kawashima, S.; Takebe, S.; Kubo, F.; Miyatsuka, T.; Kaneto, H.; Shimomura, I. A novel function of Onecut1 protein as a negative regulator of MafA gene expression. J. Biol. Chem. 2013, 288, 21648–21658.
- Du, A.; Hunter, C.S.; Murray, J.; Noble, D.; Cai, C.L.; Evans, S.M.; Stein, R.; May, C.L. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes 2009, 58, 2059–2069.
- Artner, I.; Blanchi, B.; Raum, J.C.; Guo, M.; Kaneko, T.; Cordes, S.; Sieweke, M.; Stein, R. MafB is required for islet beta cell maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 3853–3858.
- Aguayo-Mazzucato, C.; Zavacki, A.M.; Marinelarena, A.; Hollister-Lock, J.; El Khattabi, I.; Marsili, A.; Weir, G.C.; Sharma, A.; Larsen, P.R.; Bonner-Weir, S. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013, 62, 1569–1580.
- Blanchet, E.; Van de Velde, S.; Matsumura, S.; Hao, E.; LeLay, J.; Kaestner, K.; Montminy, M. Feedback inhibition of CREB signaling promotes beta cell dysfunction in insulin resistance. Cell Rep. 2015, 10, 1149–1157.
- Guo, S.; Vanderford, N.L.; Stein, R. Phosphorylation within the MafA N terminus regulates C-terminal dimerization and DNA binding. J. Biol. Chem. 2010, 285, 12655–12661.




