Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ji-Xin Tang | -- | 2167 | 2022-04-19 12:07:31 | | | |
| 2 | Rita Xu | Meta information modification | 2167 | 2022-04-20 04:42:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tang, J.; , .; Tang, J.; Deng, S.; Liu, Y. Extracellular Heat Shock Proteins. Encyclopedia. Available online: https://encyclopedia.pub/entry/21939 (accessed on 06 March 2026).
Tang J, , Tang J, Deng S, Liu Y. Extracellular Heat Shock Proteins. Encyclopedia. Available at: https://encyclopedia.pub/entry/21939. Accessed March 06, 2026.
Tang, Ji-Xin, , Jixin Tang, Shou-Long Deng, Yi-Yun Liu. "Extracellular Heat Shock Proteins" Encyclopedia, https://encyclopedia.pub/entry/21939 (accessed March 06, 2026).
Tang, J., , ., Tang, J., Deng, S., & Liu, Y. (2022, April 19). Extracellular Heat Shock Proteins. In Encyclopedia. https://encyclopedia.pub/entry/21939
Tang, Ji-Xin, et al. "Extracellular Heat Shock Proteins." Encyclopedia. Web. 19 April, 2022.
Copy Citation
Heat shock proteins (HSPs) are highly conserved stress proteins known as molecular chaperones, which are considered to be cytoplasmic proteins with functions restricted to the intracellular compartment, such as the cytoplasm or cellular organelles. However, an increasing number of observations have shown that HSPs can also be released into the extracellular matrix and can play important roles in the modulation of inflammation and immune responses.
heat shock proteins
extracellular HSPs
exosomes
inflammation
1. Introduction
Heat shock proteins (HSPs), the conserved molecular chaperone proteins, were originally assumed to be stress-responsive proteins required for cell or organism survival after exposure to thermal stress. Shortly afterwards, it was found that HSPs could be induced by a wider variety of insults, such as inflammation, ischemia, and oxidative stress, in order to protect cells from further injury or death [1][2][3][4][5]. Although they had been named heat shock proteins or heat stress proteins, many of the HSPs are ubiquitously expressed in almost all types cells even under the physiological conditions, as they are essential for maintaining cellular homeostasis, such as protein homeostasis (proteostasis) [6][7][8][9].
According to their molecular weight and the characteristic domains, eukaryotic cellullar HSPs can be divided into several classes, such as Hsp60, Hsp70, and Hsp90 chaperones. Different classes of HSPs possess specific activities and functions, and sometimes they often work together to help native protein folding and maintain correct protein conformations, and therefore maintain their biological activities [10][11][12]. Moreover, HSPs can also re-fold denatured peptides, inhibit the aggregation of incorrectly folded proteins, and assist the defective or irreversibly misfolded proteins or peptides for degradation through the proteasomal and autophagic pathways [10][13]. HSPs often function as complexes which include other HSPs, co-chaperones, various accessory proteins, as well as ATPase activity modulators [14][15][16]. In 2009, clear nomenclature for HSPs was proposed and, currently, they are divided into six major and broadly conserved families, i.e., HSP100s, HSP90s, HSP70s, HSP60s, HSP40s and small HSPs [17].
For the last 20 years, HSPs have been considered to be typical intracellular proteins since the report of the heat shock response and characterization of the 70 kD heat shock proteins. However, growing evidence suggests that HSPs can be released into the extracellular space and blood and that they play essential roles in many human diseases through the modulation of inflammation and immune responses [18][19][20][21][22].
2. HSPs Can Be Released into the Extracellular Milieu and Can Be Taken up by Other Cells
The evidence that HSPs can be released and can be taken up was first supplied by Tytell et al. in 1986, they reported that HSPs could be transferred from gila to axon [23]. Three years later, another team also reported that HSPs could be released by cultured rat embryo cells [24]. The release of these HSPs could not be inhibited by the inhibitors of the common secretory pathway, such as monensin or colchicine, but could be blocked by adding the lysine analogue aminoethyl cysteine, suggesting that HSPs were selectively released through an uncanonical mechanism of secretion [24]. At that time, these findings were regarded as potential artifacts and were neglected for many years, as these findings were against the common knowledge that HSPs were merely expressed intracellularly, since they lacked a classic secretion signal peptide in their sequence [25][26].
eHSPs attracted the attention of researchers again in the year 2000. In 2000, Srivastava et al. showed that necrotic cells but not apoptotic cells could release HSPs, such as gp96, calreticulin, hsp90, and hsp70, into the extracellular milieu, which could stimulate antigen-presenting cells (APC), such as macrophages and dendritic cells, through the nuclear factor kappa B (NF-kB) pathway to secrete several different cytokines [27]. Similarly, Calderwood et al. found that recombinant HSP70 could bind with high affinity to the monocyte surface, which could elicit a rapid monocyte intracellular calcium flux, causing activation of the NF-kB pathway and production of cytokines, such as the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 [28]. Moreover, they also found that the binding of recombinant HSP70 with monocyte could activate two different signal transduction pathways: one dependent on CD14 and intracellular calcium, leading to the production of IL-1β, IL-6, and TNF-α; and the other only dependent on intracellular calcium, merely causing the production of TNF-α [28]. These studies suggested that necrotic cells could passively release HSPs into the extracellular milieu, and that these eHSPs could stimulate monocyte activation through the nuclear factor kappa B (NF-kB) pathway to produce cytokines, and therefore, could modulate the immune system.
However, the potential role of eHSPs, such as HSP70, in modulating the immune system was challenged by several studies [29][30]. These studies assumed that the effect of eHSPs on the immune system was probably due to the contamination of bacterial lipopolysaccharide or other bacterial products, such as the exogenous antigenic peptides [29][30]. Subsequently, several teams have provided reliable evidence showing that eHSP70 was actually able to activate the immune cells, such as macrophages and monocytes [31][32]. Nowadays, growing evidence has shown that HSPs could be released by various types of cells in several ways, passively or actively, and therefore, the scientific community has fully accepted this phenomenon and more attention has been given to the function of eHSPs in many human diseases [19][20][21][33][34].
3. The Mechanism of HSP Transportation from Intracellular to the Extracellular Milieu
Whether HSPs are released passively after cellular necrosis or exported through an active mechanism independent of cellular necrosis has been debated, since they have been detected in extracellular milieu or in plasma. Basu et al. assumed that the release of HSP70 into the extracellular milieu was the consequence of cell lysis caused by necrotic but not apoptotic cell death [27]. Therefore, they supposed that HSPs were passively released by the necrotic cells due to incomplete cell membranes. However, Hightower and Guidon showed that the release of HSP70 to the extracellular milieu was not dependent on cellular necrosis, but through a selective release mechanism [24], which was further confirmed by Hunter-Lavin et al. [35]. They found that the release of Hsp70 into blood or culture medium from peripheral blood mononuclear cells was not due to cell damage, but through a nonclassical pathway involving lysosomal lipid rafts [35]. Therefore, it is very likely that HSPs can be released from cells through both passive and active pathways.
The secretion of proteins through the classic ER-Golgi pathway needs a consensus peptide signal to guide their transmembrane transport. However, most of the HSPs do not contain the consensus peptide signal. Moreover, inhibition of the ER-Golgi pathway via the typical inhibitors, such as brefeldin A, did not inhibit the release of HSP70 from living rat embryo cells [24]. These observations suggest that HSPs are probably actively exported via the nonclassic or unconventional secretory pathway but not the classic ER-Golgi pathway in living cells [36][37][38][39][40].
Unconventional protein secretion (UPS) is conducted by a complex protein secretion system, which can transport proteins, without a signal peptide or a transmembrane domain, across the plasma membrane or can even enter the extracellular milieu by bypassing the ER-Golgi pathway [41][42][43][44]. There are no unifying mechanisms for the UPS to transport the leaderless proteins [41][42]. The leaderless cargos are secreted from the cytosol to the extracellular milieu through several mechanisms, such as pore-mediated translocation across the plasma membrane, ABC transporter-based secretion, and autophagosome/endosome-based secretion [40][41][43][45][46][47] (Figure 1). Although the specific mechanisms of eHSP secretion have not been fully elucidated, all researchers know for certain is that eHSPs are transported through different mechanisms and these mechanisms may coexist and act together for the secretion of the same eHSP in one cell.
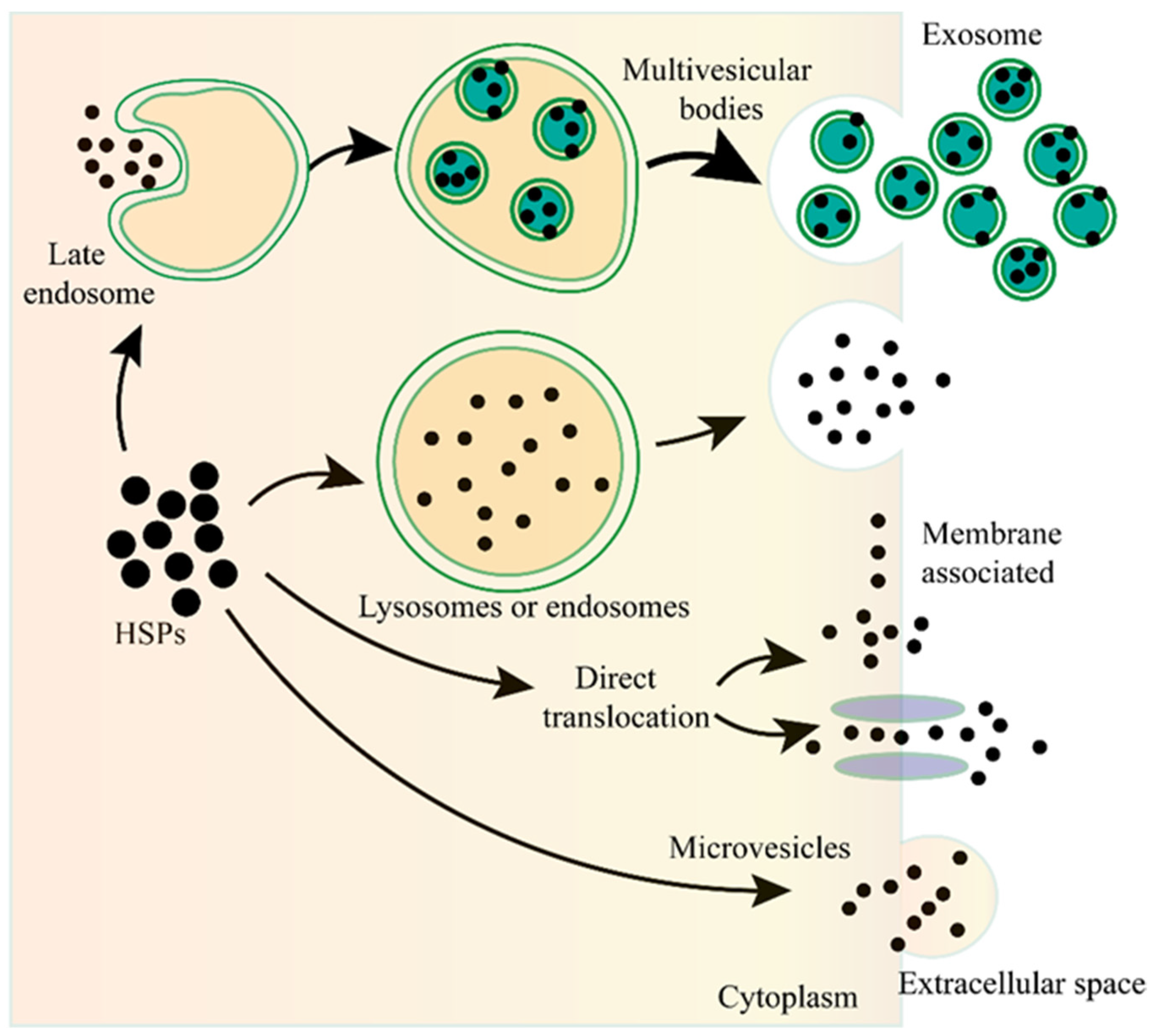
Figure 1. The unconventional secretion of Heat shock proteins (HSPs) outside the cell. After fusion of the lysosome or endosome with the plasma membrane, the HSPs can be released to the outside of the cell. HSPs captured from the cytoplasm can form vesicles, leading to the biogenesis of multivesicular bodies, and these internal vesicles are released outside the cell to become exosomes. HSPs can be directly translocated from the cytoplasm across the plasma membrane with or without the ATP binding cassette (ABC) transporter. HSPs can also be released into the extracellular space via microvesicles shed from the cell surface.
Exosomes are nano-sized extracellular vesicles (40–100 nm in diameter) that are secreted by various cell types, contain proteins, nucleic acids, or other cargos, and play essential roles in the communication between different cells [48][49][50][51][52]. As the important type of UPS, exosomes are also involved in the secretion of eHSPs [53][54][55][56][57]. The main function of HSPs is to maintain cellular proteostasis, which needs the ATP to provide energy. However, there are not enough ATP in the extracellular milieu to supply the HSPs, therefore, eHSPs cannot exert their intracellullar function to maintain protein activity. Exosomes can transport HSPs from one cell to another, therefore, this may be a novel cellular stress signal transduction pathway that can functionally compensate for the imbalanced state of the heat shock response among different cells, and therefore, maintain the organismal proteostasis [56].
4. eHSPs in the Modulation of Inflammation and Immune Responses
As mentioned above, intracellular HSPs function as molecular chaperones to maintain cellular proteostasis and play a role in cytoprotection after stressful stimuli. However, eHSPs are unable to achieve such functions due to the limitation of the environment. It is now well established that eHSPs have roles in functions that are different from the well-understood intracellular molecular chaperone role [58]. The main function of eHSPs is to modulate the immune system by activating various immune cells, such as dendritic cells (DCs), macrophages, and monocytes [59][60] (Figure 2).
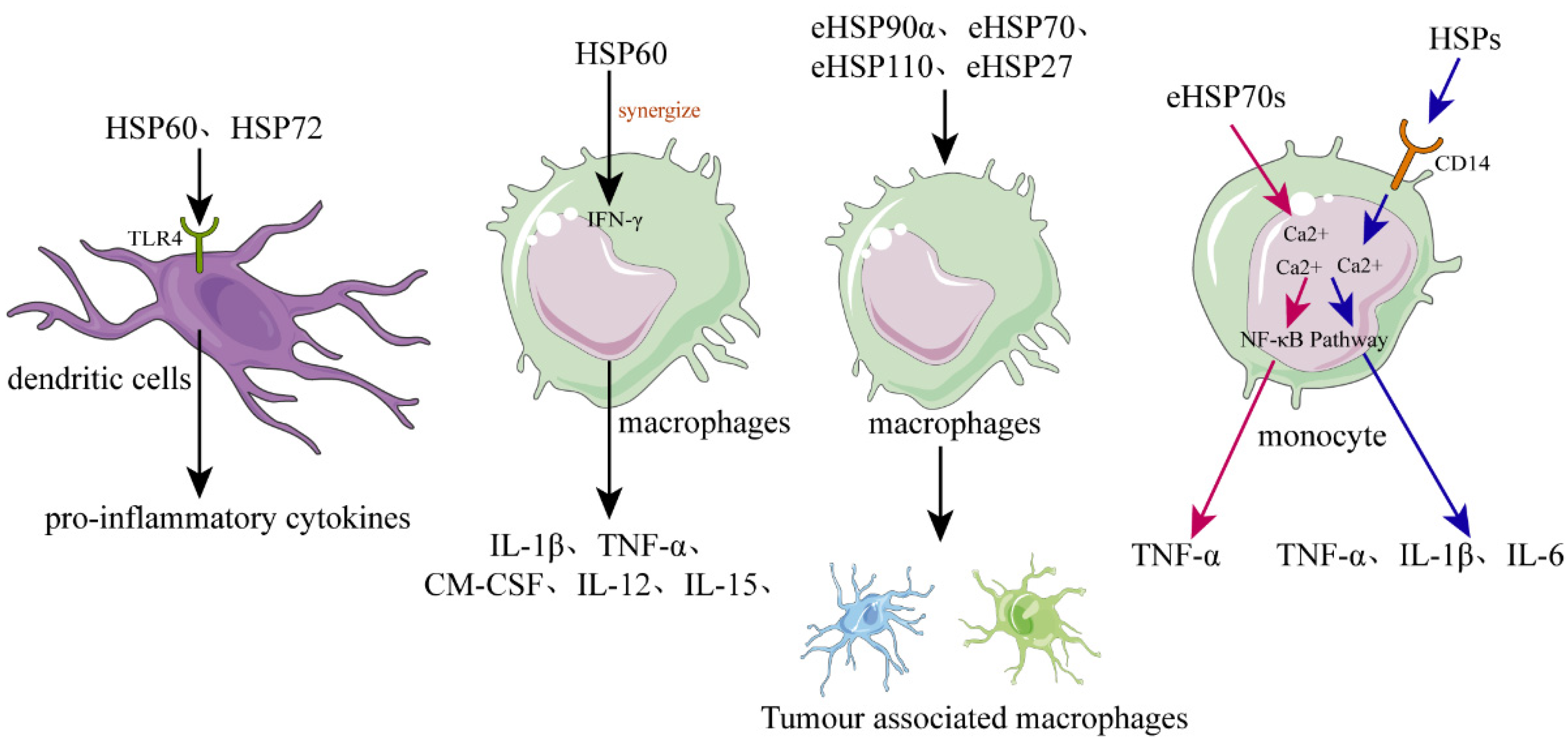
Figure 2. The role of HSPs in regulating inflammation and immune responses. In dendritic cells (DCs), recombinant human HSPs, such as HSP60 and HSP72, can promote their maturation and can activate them to secrete the proinflammatory cytokines. Released or recombinant eHSPs can stimulate macrophages to secrete various cytokines, such as IL-1β, TNF-α, GM-CSF, and IL-12, while hsp60 can synergize with IFN-γ. Various eHSPs, such as eHSP90α, eHSP70, eHSP110, and eHSP27, can stimulate the macrophages to polarize toward to TAM-like macrophages that possess immunosuppressive and proangiogenic phenotypes, promoting the progression of cancer. Regarding monocytes, they can be activated by eHSP70 probably through two different signal transduction pathways, the one pathway dependent on intracellular calcium and CD14, causing monocytes to increase the expression of IL-1β, IL-6, and TNF-α, and the other pathway only dependent on intracellular calcium resulting in increased expression of TNF-α but not IL-1β or IL-6.
4.1. eHSPs Activate DCs
DCs are a special group of immune cells that function as a bond between innate and adaptive immunity, which can take up antigens via surface receptors and process these antigens and present them to both CD8+ and CD4+ T cells [61]. Additionally, the activated DCs can also upregulate many molecules, such as the costimulatory molecules, cytokines, and chemokines; therefore, DCs are essential components of both innate and adaptive immune responses [62].
Basu et al. showed that necrotic cells could release eHSPs, such as gp96 and hsp70, which could induce DCs to upregulate the expression of antigen-presenting and co-stimulatory molecules [27]. Moreover, they demonstrated that these eHSPs activated DCs via the highly conserved NF-kB pathway [27]. The activation of antigen-presenting cells (APC) by necrotic cell-released eHSPs may be an excellent mechanism for an organism to respond to tissue cell death caused by various internal and external stimuli. In addition, recombinant human HSP60 and human inducible HSP72 can also promote human DC maturation and activate DCs to secrete the proinflammatory cytokines [63][64]. Further study has shown that these HSPs could be specifically internalized by the CD14(-), Toll-like receptor 4(-) monocyte-derived DCs via the receptor-mediated endocytosis [65]. Released or recombinant eHSPs promoted DCs maturation and cytokines secretion, probably through the Toll-like receptor 4 dependent signaling pathway, as DCs of C3H/HeJ mice deficiency of a functional Toll-like receptor 4 had no response to HSP60 stimulation [66].
4.2. eHSPs Activate Macrophages and Modulate Their Polarization
As the representative of the first line of defense in innate immune responses, macrophages play an essential role in the regulation of host inflammation and immune responses by performing phagocytic activity, delivering the proinflammatory and anti-inflammatory cytokines, and shaping the tissue microenvironment [67][68][69]. Macrophages can be polarized to M1 or M2 phenotypes according to the microenvironmental stimuli. M1 macrophages produce the proinflammatory cytokines, such as IL-6, IL-12, and TNFα, whereas, the M2 macrophages release the anti-inflammatory cytokines, such as IL-10 and TGFβ.
Released or recombinant eHSPs can stimulate macrophages to secrete various cytokines, such as IL-1β, TNF-α, GM-CSF, and IL-12 [27][70][71]. For example, human HSP60 can be recognized by macrophages, and can cause the rapid release of TNF-α or nitric oxide [70]. Moreover, the proinflammatory macrophage response of hsp60 can synergize with IFN-γ, causing the macrophage to express the Th1-promoting cytokines, such as IL-12 and IL-15 [70]. Therefore, necrotic cell-released eHSPs may be a danger signal for the innate immune system that plays an important role in the chronic Th1-dependent tissue inflammation.
Tumor-associated macrophage (TAM), recruited and activated by cancer cells, appears in the advanced stages of cancer progression and shows the M2-like phenotype [72][73]. TAMs can promote cancer growth through providing an immunosuppressive microenvironment for cancer cells [74]. Various eHSPs, such as eHSP90α, eHSP70, eHSP110, and eHSP27, can stimulate macrophages to polarize toward TAM-like macrophages possessing immunosuppressive and proangiogenic phenotypes, and therefore, contribute to the continue progression of cancer [75][76][77][78][79][80].
References
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677.
- Pockley, A.G. Heat shock proteins as regulators of the immune response. Lancet 2003, 362, 469–476.
- Zhou, X.C.; Zhang, Z.H.; Hu, Z.Y.; Zou, R.J.; Liu, Y.X. Expression of Hsp70-2 in rhesus monkey testis during germ cell apoptosis induced by testosterone undecanoate. Contraception 2002, 66, 377–382.
- Guo, C.X.; Ma, J.; Zhou, X.C.; Liu, Y.X. Expression of HSP70-2 gene during germ cell apoptosis in rat unilateral cryptorchid testes. Arch. Androl. 2001, 46, 109–115.
- Zhou, X.C.; Han, X.B.; Hu, Z.Y.; Zhou, R.J.; Liu, Y.X. Expression of Hsp70-2 in unilateral cryptorchid testis of rhesus monkey during germ cell apoptosis. Endocrine 2001, 16, 89–95.
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266.
- Reinle, K.; Mogk, A.; Bukau, B. The Diverse Functions of Small Heat Shock Proteins in the Proteostasis Network. J. Mol. Biol. 2021, 434, 167157.
- Vertti-Quintero, N.; Berger, S.; Casadevall, I.S.X.; Statzer, C.; Annis, J.; Ruppen, P.; Stavrakis, S.; Ewald, C.Y. Stochastic and Age-Dependent Proteostasis Decline Underlies Heterogeneity in Heat-Shock Response Dynamics. Small 2021, 17, e2102145.
- Yu, C.; Leung, S.K.P.; Zhang, W.; Lai, L.T.F. Structural basis of substrate recognition and thermal protection by a small heat shock protein. Nat. Commun. 2021, 12, 3007.
- Hightower, L.E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991, 66, 191–197.
- Zatsepina, O.G.; Evgen’ev, M.B.; Garbuz, D.G. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells 2021, 10, 1638.
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970.
- Chaudhury, S.; Keegan, B.M.; Blagg, B.S. The role and therapeutic potential of Hsp90, Hsp70, and smaller heat shock proteins in peripheral and central neuropathies. Med. Res. Rev. 2021, 41, 202–222.
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120.
- Noddings, C.M.; Wang, R.Y.; Johnson, J.L.; Agard, D.A. Structure of Hsp90-p23-GR reveals the Hsp90 client-remodelling mechanism. Nature 2022, 601, 465–469.
- Wang, R.Y.; Noddings, C.M.C.M.N.; Kirschke, E.; Myasnikov, A.G.; Johnson, J.L.; Agard, D.A. Structure of Hsp90-Hsp70-Hop-GR reveals the Hsp90 client-loading mechanism. Nature 2022, 601, 460–464.
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111.
- Seclì, L.; Fusella, F.; Avalle, L.; Brancaccio, M. The dark-side of the outside: How extracellular heat shock proteins promote cancer. Cell. Mol. Life Sci. 2021, 78, 4069–4083.
- Tanguy, J.; Pommerolle, L.; Garrido, C.; Kolb, M.; Bonniaud, P.; Goirand, F.; Bellaye, P.S. Extracellular Heat Shock Proteins as Therapeutic Targets and Biomarkers in Fibrosing Interstitial Lung Diseases. Int. J. Mol. Sci. 2021, 22, 9316.
- Van den Broek, B.; Wuyts, C.; Irobi, J. Extracellular vesicle-associated small heat shock proteins as therapeutic agents in neurodegenerative diseases and beyond. Adv. Drug Deliv. Rev. 2021, 179, 114009.
- Caruso Bavisotto, C.; Marino Gammazza, A.; Campanella, C.; Bucchieri, F.; Cappello, F. Extracellular heat shock proteins in cancer: From early diagnosis to new therapeutic approach. Semin. Cancer Biol. 2021.
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354.
- Tytell, M.; Greenberg, S.G.; Lasek, R.J. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986, 363, 161–164.
- Hightower, L.E.; Guidon, P.T., Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell Physiol. 1989, 138, 257–266.
- Mambula, S.S.; Stevenson, M.A.; Ogawa, K.; Calderwood, S.K. Mechanisms for Hsp70 secretion: Crossing membranes without a leader. Methods 2007, 43, 168–175.
- Mambula, S.S.; Calderwood, S.K. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 2006, 177, 7849–7857.
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546.
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442.
- Gao, B.; Tsan, M.F. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J. Biol. Chem. 2003, 278, 174–179.
- Bendz, H.; Marincek, B.C.; Momburg, F.; Ellwart, J.W.; Issels, R.D.; Nelson, P.J.; Noessner, E. Calcium signaling in dendritic cells by human or mycobacterial Hsp70 is caused by contamination and is not required for Hsp70-mediated enhancement of cross-presentation. J. Biol. Chem. 2008, 283, 26477–26483.
- Zheng, H.; Nagaraja, G.M.; Kaur, P.; Asea, E.E.; Asea, A. Chaperokine function of recombinant Hsp72 produced in insect cells using a baculovirus expression system is retained. J. Biol. Chem. 2010, 285, 349–356.
- Vega, V.L.; Rodríguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; De Maio, A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 2008, 180, 4299–4307.
- Xiong, J.; Li, Y.; Tan, X.; Fu, L. Small Heat Shock Proteins in Cancers: Functions and Therapeutic Potential for Cancer Therapy. Int J Mol Sci. 2020, 21, 6611.
- Albakova, Z.; Siam, M.K.S.; Sacitharan, P.K.; Ziganshin, R.H.; Ryazantsev, D.Y.; Sapozhnikov, A.M. Extracellular heat shock proteins and cancer: New perspectives. Transl. Oncol. 2021, 14, 100995.
- Hunter-Lavin, C.; Davies, E.L.; Bacelar, M.M.; Marshall, M.J.; Andrew, S.M.; Williams, J.H. Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2004, 324, 511–517.
- Nickel, W.; Seedorf, M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 2008, 24, 287–308.
- Nickel, W. Unconventional secretory routes: Direct protein export across the plasma membrane of mammalian cells. Traffic 2005, 6, 607–614.
- Dimou, E.; Nickel, W. Unconventional mechanisms of eukaryotic protein secretion. Curr. Biol. 2018, 28, R406–R410.
- Dimou, E.; Cosentino, K.; Platonova, E.; Uris Ros, M.S.; Kashyap, P.; Katsinelos, T.; Wegehingel, S.; Noé, F.; García-Sáez, A.J.; Ewers, H.; et al. Single event visualization of unconventional secretion of FGF2. J. Cell Biol. 2019, 218, 683–699.
- Zhang, M.; Liu, L.; Lin, X.; Wang, Y.; Li, Y.; Guo, Q.; Li, S.; Sun, Y.; Tao, X.; Zhang, D.; et al. A Translocation Pathway for Vesicle-Mediated Unconventional Protein Secretion. Cell 2020, 181, 637–652.e615.
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240.
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009, 10, 148–155.
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. Embo J. 2011, 30, 4701–4711.
- Urano, Y.; Mori, C.; Fuji, A.; Konno, K.; Yamamoto, T.; Yashirogi, S.; Ando, M.; Saito, Y.; Noguchi, N. 6-Hydroxydopamine induces secretion of PARK7/DJ-1 via autophagy-based unconventional secretory pathway. Autophagy 2018, 14, 1943–1958.
- Saraste, J.; Prydz, K. Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space. Cells 2021, 10, 503.
- Wang, Z.; Zhou, H.; Zheng, H.; Zhou, X.; Shen, G.; Teng, X.; Liu, X.; Zhang, J.; Wei, X.; Hu, Z.; et al. Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy 2021, 17, 529–552.
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535.
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934.
- Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2021, 20, 6–7.
- Robson, A. Exosome-derived microRNAs improve cardiac function. Nat. Rev. Cardiol. 2021, 18, 150–151.
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47.
- Tang, X.H.; Guo, T.; Gao, X.Y.; Wu, X.L.; Xing, X.F.; Ji, J.F.; Li, Z.Y. Exosome-derived noncoding RNAs in gastric cancer: Functions and clinical applications. Mol. Cancer 2021, 20, 99.
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355.
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005, 118, 3631–3638.
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247.
- Takeuchi, T.; Suzuki, M.; Fujikake, N.; Popiel, H.A.; Kikuchi, H.; Futaki, S.; Wada, K.; Nagai, Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. USA 2015, 112, E2497–E2506.
- Tang, X.; Chang, C.; Guo, J.; Lincoln, V.; Liang, C.; Chen, M.; Woodley, D.T.; Li, W. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour—Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci. Rep. 2019, 9, 15108.
- De Maio, A.; Vazquez, D. Extracellular heat shock proteins: A new location, a new function. Shock 2013, 40, 239–246.
- De Maio, A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 2011, 16, 235–249.
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588.
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68.
- Carenza, C.; Calcaterra, F.; Oriolo, F.; Di Vito, C.; Ubezio, M.; Della Porta, M.G.; Mavilio, D.; Della Bella, S. Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front. Immunol. 2019, 10, 1325.
- Bethke, K.; Staib, F.; Distler, M.; Schmitt, U.; Jonuleit, H.; Enk, A.H.; Galle, P.R.; Heike, M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: Superiority of HSP60. J. Immunol. 2002, 169, 6141–6148.
- Kuppner, M.C.; Gastpar, R.; Gelwer, S.; Nössner, E.; Ochmann, O.; Scharner, A.; Issels, R.D. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur. J. Immunol. 2001, 31, 1602–1609.
- Lipsker, D.; Ziylan, U.; Spehner, D.; Proamer, F.; Bausinger, H.; Jeannin, P.; Salamero, J.; Bohbot, A.; Cazenave, J.P.; Drillien, R.; et al. Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. Eur. J. Immunol. 2002, 32, 322–332.
- Flohé, S.B.; Brüggemann, J.; Lendemans, S.; Nikulina, M.; Meierhoff, G.; Flohé, S.; Kolb, H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J. Immunol. 2003, 170, 2340–2348.
- Dukhinova, M.; Kokinos, E.; Kuchur, P.; Komissarov, A.; Shtro, A. Macrophage-derived cytokines in pneumonia: Linking cellular immunology and genetics. Cytokine Growth Factor Rev. 2021, 59, 46–61.
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714.
- Liang, S.; Wu, Y.S.; Li, D.Y.; Tang, J.X.; Liu, H.F. Autophagy in Viral Infection and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 766142.
- Chen, W.; Syldath, U.; Bellmann, K.; Burkart, V.; Kolb, H. Human 60-kDa heat-shock protein: A danger signal to the innate immune system. J. Immunol. 1999, 162, 3212–3219.
- Quintana, F.J.; Cohen, I.R. The HSP60 immune system network. Trends Immunol. 2011, 32, 89–95.
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559.
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Corrigendum: Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2021, 12, 775758.
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021, 11, 2892–2916.
- Chen, C.C.; Chen, L.L.; Li, C.P.; Hsu, Y.T.; Jiang, S.S.; Fan, C.S.; Chua, K.V.; Huang, S.X.; Shyr, Y.M.; Chen, L.T.; et al. Myeloid-derived macrophages and secreted HSP90α induce pancreatic ductal adenocarcinoma development. Oncoimmunology 2018, 7, e1424612.
- Chua, K.V.; Fan, C.S.; Chen, C.C.; Chen, L.L.; Hsieh, S.C.; Huang, T.S. Octyl Gallate Induces Pancreatic Ductal Adenocarcinoma Cell Apoptosis and Suppresses Endothelial-Mesenchymal Transition-Promoted M2-Macrophages, HSP90α Secretion, and Tumor Growth. Cells 2019, 9, 91.
- Fan, C.S.; Chen, L.L.; Hsu, T.A.; Chen, C.C.; Chua, K.V.; Li, C.P.; Huang, T.S. Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2019, 12, 138.
- Kaczmarek, M.; Lagiedo, M.; Masztalerz, A.; Kozlowska, M.; Nowicka, A.; Brajer, B.; Batura-Gabryel, H.; Sikora, J. Concentrations of SP-A and HSP70 are associated with polarization of macrophages in pleural effusions of non-small cell lung cancer. Immunobiology 2018, 223, 200–209.
- Berthenet, K.; Boudesco, C.; Collura, A.; Svrcek, M.; Richaud, S.; Hammann, A.; Causse, S.; Yousfi, N.; Wanherdrick, K.; Duplomb, L.; et al. Extracellular HSP110 skews macrophage polarization in colorectal cancer. Oncoimmunology 2016, 5, e1170264.
- Banerjee, S.; Lin, C.F.; Skinner, K.A.; Schiffhauer, L.M.; Peacock, J.; Hicks, D.G.; Redmond, E.M.; Morrow, D.; Huston, A.; Shayne, M.; et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011, 71, 318–327.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
20 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





