Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Heat shock proteins (HSPs) are highly conserved stress proteins known as molecular chaperones, which are considered to be cytoplasmic proteins with functions restricted to the intracellular compartment, such as the cytoplasm or cellular organelles. However, an increasing number of observations have shown that HSPs can also be released into the extracellular matrix and can play important roles in the modulation of inflammation and immune responses.
- heat shock proteins
- extracellular HSPs
- exosomes
- inflammation
1. Introduction
Heat shock proteins (HSPs), the conserved molecular chaperone proteins, were originally assumed to be stress-responsive proteins required for cell or organism survival after exposure to thermal stress. Shortly afterwards, it was found that HSPs could be induced by a wider variety of insults, such as inflammation, ischemia, and oxidative stress, in order to protect cells from further injury or death [1][2][3][4][5]. Although they had been named heat shock proteins or heat stress proteins, many of the HSPs are ubiquitously expressed in almost all types cells even under the physiological conditions, as they are essential for maintaining cellular homeostasis, such as protein homeostasis (proteostasis) [6][7][8][9].
According to their molecular weight and the characteristic domains, eukaryotic cellullar HSPs can be divided into several classes, such as Hsp60, Hsp70, and Hsp90 chaperones. Different classes of HSPs possess specific activities and functions, and sometimes they often work together to help native protein folding and maintain correct protein conformations, and therefore maintain their biological activities [10][11][12]. Moreover, HSPs can also re-fold denatured peptides, inhibit the aggregation of incorrectly folded proteins, and assist the defective or irreversibly misfolded proteins or peptides for degradation through the proteasomal and autophagic pathways [10][13]. HSPs often function as complexes which include other HSPs, co-chaperones, various accessory proteins, as well as ATPase activity modulators [14][15][16]. In 2009, clear nomenclature for HSPs was proposed and, currently, they are divided into six major and broadly conserved families, i.e., HSP100s, HSP90s, HSP70s, HSP60s, HSP40s and small HSPs [17].
For the last 20 years, HSPs have been considered to be typical intracellular proteins since the report of the heat shock response and characterization of the 70 kD heat shock proteins. However, growing evidence suggests that HSPs can be released into the extracellular space and blood and that they play essential roles in many human diseases through the modulation of inflammation and immune responses [18][19][20][21][22].
2. HSPs Can Be Released into the Extracellular Milieu and Can Be Taken up by Other Cells
The evidence that HSPs can be released and can be taken up was first supplied by Tytell et al. in 1986, they reported that HSPs could be transferred from gila to axon [23]. Three years later, another team also reported that HSPs could be released by cultured rat embryo cells [24]. The release of these HSPs could not be inhibited by the inhibitors of the common secretory pathway, such as monensin or colchicine, but could be blocked by adding the lysine analogue aminoethyl cysteine, suggesting that HSPs were selectively released through an uncanonical mechanism of secretion [24]. At that time, these findings were regarded as potential artifacts and were neglected for many years, as these findings were against the common knowledge that HSPs were merely expressed intracellularly, since they lacked a classic secretion signal peptide in their sequence [25][26].
eHSPs attracted the attention of researchers again in the year 2000. In 2000, Srivastava et al. showed that necrotic cells but not apoptotic cells could release HSPs, such as gp96, calreticulin, hsp90, and hsp70, into the extracellular milieu, which could stimulate antigen-presenting cells (APC), such as macrophages and dendritic cells, through the nuclear factor kappa B (NF-kB) pathway to secrete several different cytokines [27]. Similarly, Calderwood et al. found that recombinant HSP70 could bind with high affinity to the monocyte surface, which could elicit a rapid monocyte intracellular calcium flux, causing activation of the NF-kB pathway and production of cytokines, such as the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 [28]. Moreover, they also found that the binding of recombinant HSP70 with monocyte could activate two different signal transduction pathways: one dependent on CD14 and intracellular calcium, leading to the production of IL-1β, IL-6, and TNF-α; and the other only dependent on intracellular calcium, merely causing the production of TNF-α [28]. These studies suggested that necrotic cells could passively release HSPs into the extracellular milieu, and that these eHSPs could stimulate monocyte activation through the nuclear factor kappa B (NF-kB) pathway to produce cytokines, and therefore, could modulate the immune system.
However, the potential role of eHSPs, such as HSP70, in modulating the immune system was challenged by several studies [29][30]. These studies assumed that the effect of eHSPs on the immune system was probably due to the contamination of bacterial lipopolysaccharide or other bacterial products, such as the exogenous antigenic peptides [29][30]. Subsequently, several teams have provided reliable evidence showing that eHSP70 was actually able to activate the immune cells, such as macrophages and monocytes [31][32]. Nowadays, growing evidence has shown that HSPs could be released by various types of cells in several ways, passively or actively, and therefore, the scientific community has fully accepted this phenomenon and more attention has been given to the function of eHSPs in many human diseases [19][20][21][33][34].
3. The Mechanism of HSP Transportation from Intracellular to the Extracellular Milieu
Whether HSPs are released passively after cellular necrosis or exported through an active mechanism independent of cellular necrosis has been debated, since they have been detected in extracellular milieu or in plasma. Basu et al. assumed that the release of HSP70 into the extracellular milieu was the consequence of cell lysis caused by necrotic but not apoptotic cell death [27]. Therefore, they supposed that HSPs were passively released by the necrotic cells due to incomplete cell membranes. However, Hightower and Guidon showed that the release of HSP70 to the extracellular milieu was not dependent on cellular necrosis, but through a selective release mechanism [24], which was further confirmed by Hunter-Lavin et al. [35]. They found that the release of Hsp70 into blood or culture medium from peripheral blood mononuclear cells was not due to cell damage, but through a nonclassical pathway involving lysosomal lipid rafts [35]. Therefore, it is very likely that HSPs can be released from cells through both passive and active pathways.
The secretion of proteins through the classic ER-Golgi pathway needs a consensus peptide signal to guide their transmembrane transport. However, most of the HSPs do not contain the consensus peptide signal. Moreover, inhibition of the ER-Golgi pathway via the typical inhibitors, such as brefeldin A, did not inhibit the release of HSP70 from living rat embryo cells [24]. These observations suggest that HSPs are probably actively exported via the nonclassic or unconventional secretory pathway but not the classic ER-Golgi pathway in living cells [36][37][38][39][40].
Unconventional protein secretion (UPS) is conducted by a complex protein secretion system, which can transport proteins, without a signal peptide or a transmembrane domain, across the plasma membrane or can even enter the extracellular milieu by bypassing the ER-Golgi pathway [41][42][43][44]. There are no unifying mechanisms for the UPS to transport the leaderless proteins [41][42]. The leaderless cargos are secreted from the cytosol to the extracellular milieu through several mechanisms, such as pore-mediated translocation across the plasma membrane, ABC transporter-based secretion, and autophagosome/endosome-based secretion [40][41][43][45][46][47] (Figure 1). Although the specific mechanisms of eHSP secretion have not been fully elucidated, all researchers know for certain is that eHSPs are transported through different mechanisms and these mechanisms may coexist and act together for the secretion of the same eHSP in one cell.
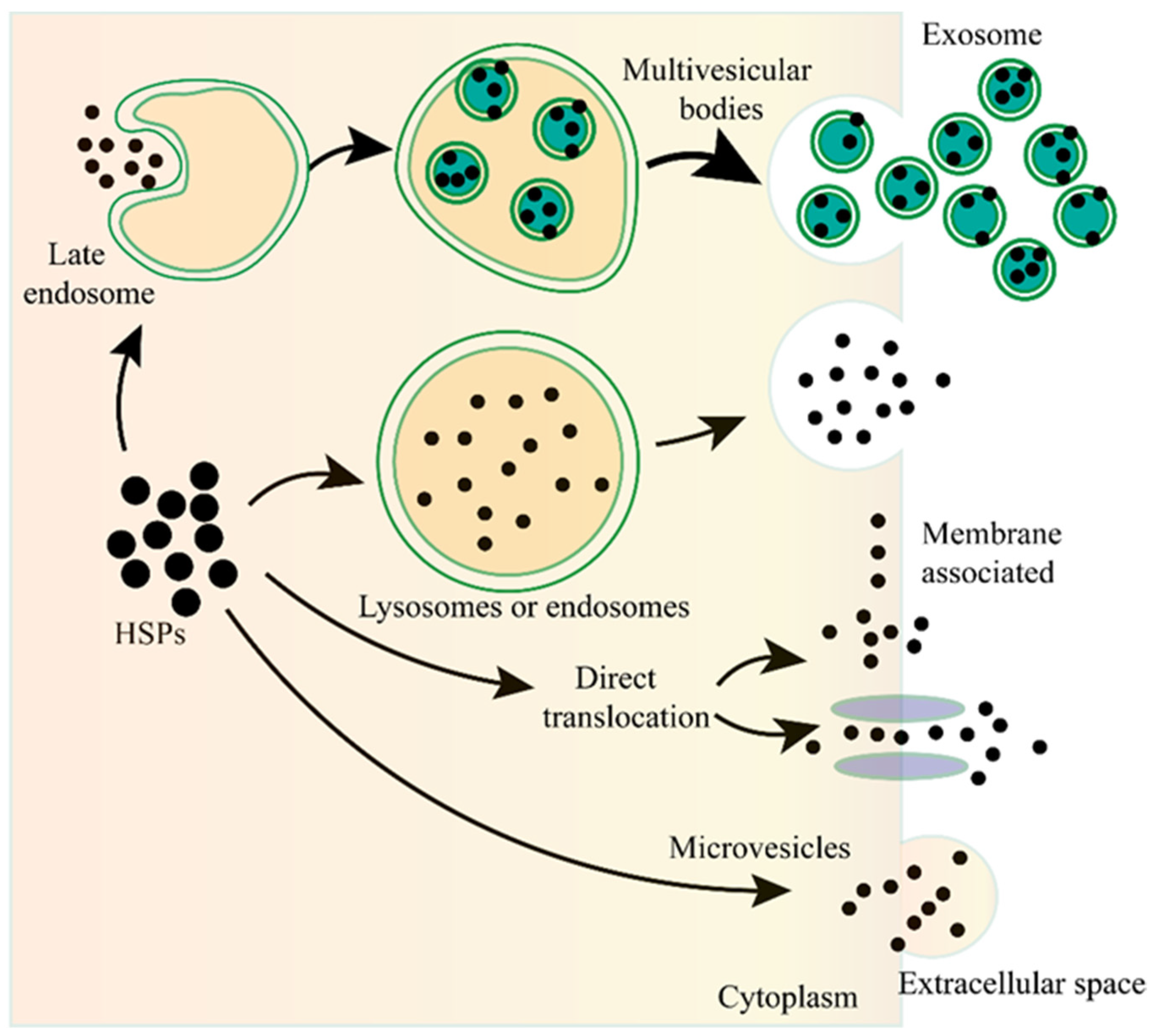
Figure 1. The unconventional secretion of Heat shock proteins (HSPs) outside the cell. After fusion of the lysosome or endosome with the plasma membrane, the HSPs can be released to the outside of the cell. HSPs captured from the cytoplasm can form vesicles, leading to the biogenesis of multivesicular bodies, and these internal vesicles are released outside the cell to become exosomes. HSPs can be directly translocated from the cytoplasm across the plasma membrane with or without the ATP binding cassette (ABC) transporter. HSPs can also be released into the extracellular space via microvesicles shed from the cell surface.
Exosomes are nano-sized extracellular vesicles (40–100 nm in diameter) that are secreted by various cell types, contain proteins, nucleic acids, or other cargos, and play essential roles in the communication between different cells [48][49][50][51][52]. As the important type of UPS, exosomes are also involved in the secretion of eHSPs [53][54][55][56][57]. The main function of HSPs is to maintain cellular proteostasis, which needs the ATP to provide energy. However, there are not enough ATP in the extracellular milieu to supply the HSPs, therefore, eHSPs cannot exert their intracellullar function to maintain protein activity. Exosomes can transport HSPs from one cell to another, therefore, this may be a novel cellular stress signal transduction pathway that can functionally compensate for the imbalanced state of the heat shock response among different cells, and therefore, maintain the organismal proteostasis [56].
4. eHSPs in the Modulation of Inflammation and Immune Responses
As mentioned above, intracellular HSPs function as molecular chaperones to maintain cellular proteostasis and play a role in cytoprotection after stressful stimuli. However, eHSPs are unable to achieve such functions due to the limitation of the environment. It is now well established that eHSPs have roles in functions that are different from the well-understood intracellular molecular chaperone role [58]. The main function of eHSPs is to modulate the immune system by activating various immune cells, such as dendritic cells (DCs), macrophages, and monocytes [59][60] (Figure 2).
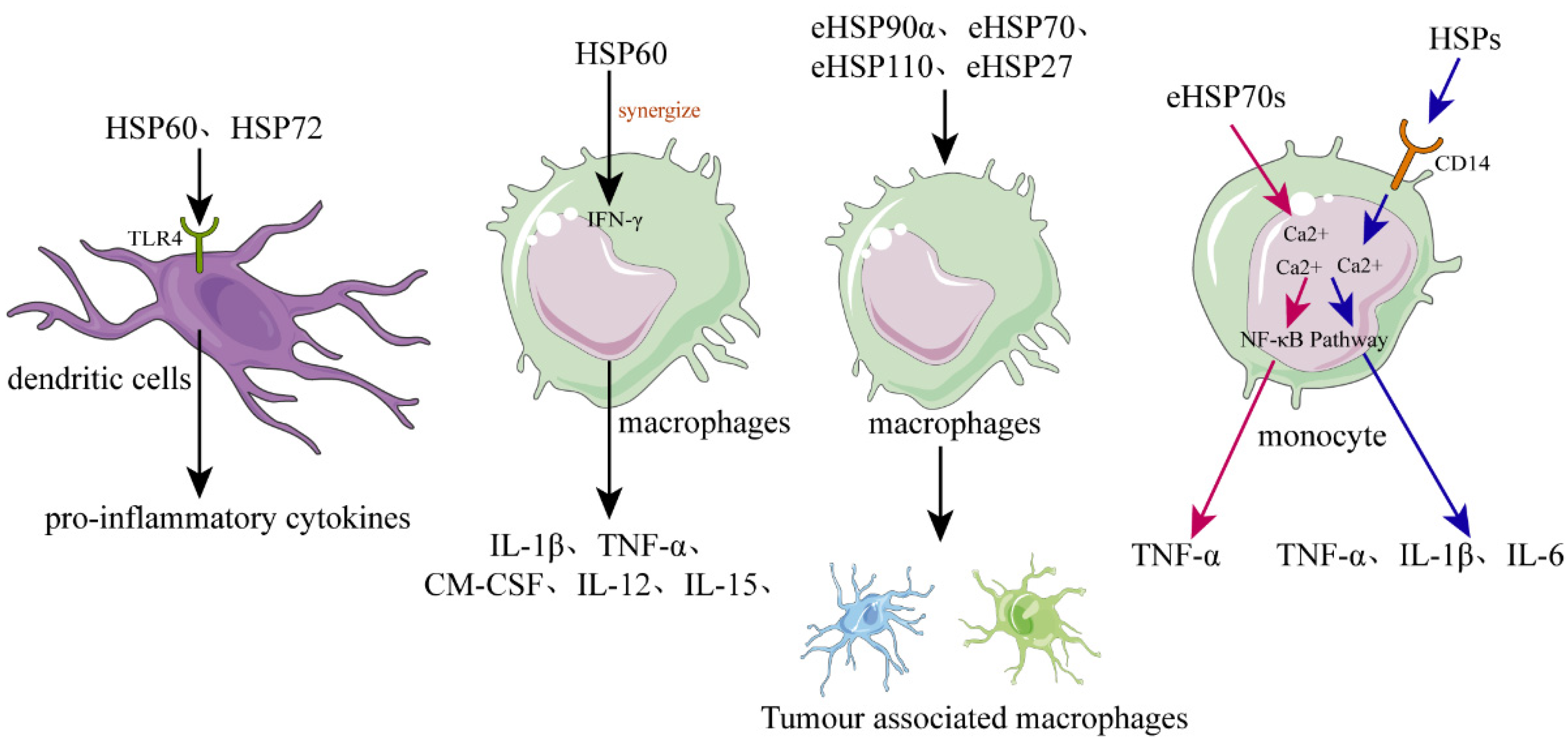
Figure 2. The role of HSPs in regulating inflammation and immune responses. In dendritic cells (DCs), recombinant human HSPs, such as HSP60 and HSP72, can promote their maturation and can activate them to secrete the proinflammatory cytokines. Released or recombinant eHSPs can stimulate macrophages to secrete various cytokines, such as IL-1β, TNF-α, GM-CSF, and IL-12, while hsp60 can synergize with IFN-γ. Various eHSPs, such as eHSP90α, eHSP70, eHSP110, and eHSP27, can stimulate the macrophages to polarize toward to TAM-like macrophages that possess immunosuppressive and proangiogenic phenotypes, promoting the progression of cancer. Regarding monocytes, they can be activated by eHSP70 probably through two different signal transduction pathways, the one pathway dependent on intracellular calcium and CD14, causing monocytes to increase the expression of IL-1β, IL-6, and TNF-α, and the other pathway only dependent on intracellular calcium resulting in increased expression of TNF-α but not IL-1β or IL-6.
4.1. eHSPs Activate DCs
DCs are a special group of immune cells that function as a bond between innate and adaptive immunity, which can take up antigens via surface receptors and process these antigens and present them to both CD8+ and CD4+ T cells [61]. Additionally, the activated DCs can also upregulate many molecules, such as the costimulatory molecules, cytokines, and chemokines; therefore, DCs are essential components of both innate and adaptive immune responses [62].
Basu et al. showed that necrotic cells could release eHSPs, such as gp96 and hsp70, which could induce DCs to upregulate the expression of antigen-presenting and co-stimulatory molecules [27]. Moreover, they demonstrated that these eHSPs activated DCs via the highly conserved NF-kB pathway [27]. The activation of antigen-presenting cells (APC) by necrotic cell-released eHSPs may be an excellent mechanism for an organism to respond to tissue cell death caused by various internal and external stimuli. In addition, recombinant human HSP60 and human inducible HSP72 can also promote human DC maturation and activate DCs to secrete the proinflammatory cytokines [63][64]. Further study has shown that these HSPs could be specifically internalized by the CD14(-), Toll-like receptor 4(-) monocyte-derived DCs via the receptor-mediated endocytosis [65]. Released or recombinant eHSPs promoted DCs maturation and cytokines secretion, probably through the Toll-like receptor 4 dependent signaling pathway, as DCs of C3H/HeJ mice deficiency of a functional Toll-like receptor 4 had no response to HSP60 stimulation [66].
4.2. eHSPs Activate Macrophages and Modulate Their Polarization
As the representative of the first line of defense in innate immune responses, macrophages play an essential role in the regulation of host inflammation and immune responses by performing phagocytic activity, delivering the proinflammatory and anti-inflammatory cytokines, and shaping the tissue microenvironment [67][68][69]. Macrophages can be polarized to M1 or M2 phenotypes according to the microenvironmental stimuli. M1 macrophages produce the proinflammatory cytokines, such as IL-6, IL-12, and TNFα, whereas, the M2 macrophages release the anti-inflammatory cytokines, such as IL-10 and TGFβ.
Released or recombinant eHSPs can stimulate macrophages to secrete various cytokines, such as IL-1β, TNF-α, GM-CSF, and IL-12 [27][70][71]. For example, human HSP60 can be recognized by macrophages, and can cause the rapid release of TNF-α or nitric oxide [70]. Moreover, the proinflammatory macrophage response of hsp60 can synergize with IFN-γ, causing the macrophage to express the Th1-promoting cytokines, such as IL-12 and IL-15 [70]. Therefore, necrotic cell-released eHSPs may be a danger signal for the innate immune system that plays an important role in the chronic Th1-dependent tissue inflammation.
Tumor-associated macrophage (TAM), recruited and activated by cancer cells, appears in the advanced stages of cancer progression and shows the M2-like phenotype [72][73]. TAMs can promote cancer growth through providing an immunosuppressive microenvironment for cancer cells [74]. Various eHSPs, such as eHSP90α, eHSP70, eHSP110, and eHSP27, can stimulate macrophages to polarize toward TAM-like macrophages possessing immunosuppressive and proangiogenic phenotypes, and therefore, contribute to the continue progression of cancer [75][76][77][78][79][80].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27072361
This entry is offline, you can click here to edit this entry!
