
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hasina Masha Aziz | -- | 2223 | 2022-04-16 16:48:49 | | | |
| 2 | Paweł Tomasz Matusik | + 13 word(s) | 2236 | 2022-04-16 23:40:12 | | | | |
| 3 | Catherine Yang | -8 word(s) | 2228 | 2022-04-18 04:17:49 | | | | |
| 4 | Catherine Yang | Meta information modification | 2228 | 2022-04-18 04:20:17 | | |
Video Upload Options
Brugada syndrome (BrS) is a primary electrical disease associated with arrhythmias and an elevated risk of sudden cardiac death (SCD). It was described by Pedro and Josep Brugada in 1992 as a syndrome comprised of “right bundle branch block, persistent ST segment elevation and SCD”. The prevalence of BrS electrocardiogram (ECG) patterns differs largely among various regions and populations of the world. Patients with BrS are considered symptomatic if they have history of aborted SCD, ventricular fibrillation (VF), sustained ventricular tachycardia (VT), or syncope. BrS usually presents during the third or fourth decade of life, and about 63% of patients are asymptomatic at diagnosis. However, syncope or major arrhythmic events (MAE) can occur at any age, or SCD may even present as the first event. BrS contributes towards sudden infant death syndrome, SCD in children, and is estimated to cause about 20% of all SCDs in individuals with anatomically normal cardiac structures.
1. Pathogenesis of BrS
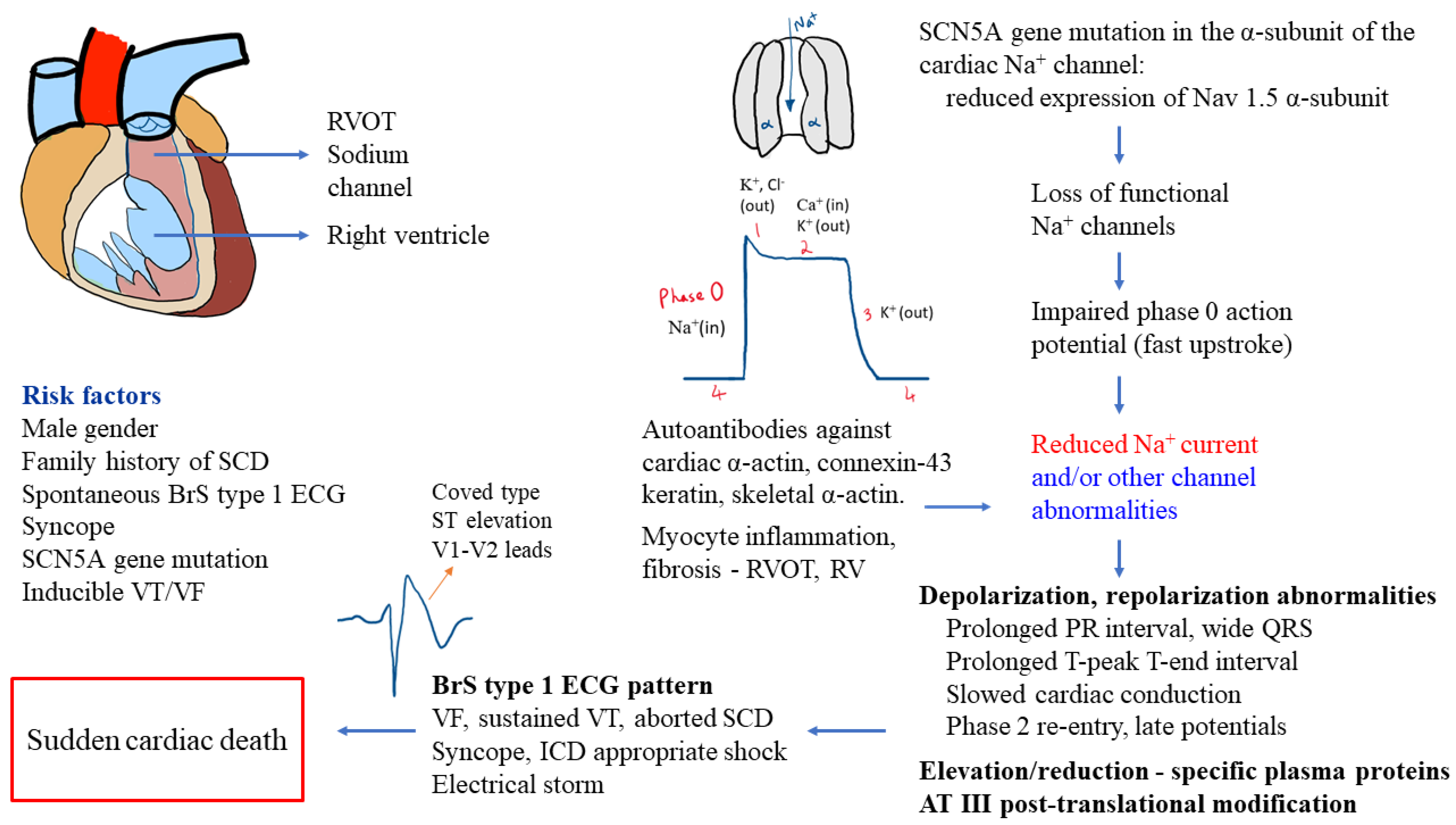
2. Diagnostics and Risk Stratification
2.1. Diagnostics in BrS
2.2. Risk Stratification in BrS
3. Treatment of Patients with BrS
References
- Sieira, J.; Brugada, P. The definition of the Brugada syndrome. Eur. Heart J. 2017, 38, 3029–3034.
- Mizusawa, Y.; Wilde, A.A.M. Brugada syndrome. Circ. Arrhythm Electrophysiol. 2012, 5, 606–616.
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Riera, A.R.P.; et al. Brugada syndrome: Report of the second consensus conference. Circulation 2005, 111, 659–670.
- Alings, M.; Wilde, A. “Brugada” syndrome. clinical data and suggested pathophysiological mechanism. Circulation 1999, 99, 666–673.
- Matusik, P.T.; Rydlewska, A.; Pudło, J.; Podolec, J.; Lelakowski, J.; Podolec, P. Brugada syndrome: New concepts and algorithms in management (RCD code: V 1A.1). J. Rare Cardiovasc. Dis. 2018, 3, 151–160.
- Chatterjee, D.; Pieroni, M.; Fatah, M.; Charpentier, F.; Cunningham, K.S.; Spears, D.A.; Chatterjee, D.; Suna, G.; Bos, J.M.; Ackerman, M.J.; et al. An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. Eur. Heart J. 2020, 41, 2878A–2890A.
- Rattanawong, P.; Kewcharoen, J.; Techorueangwiwat, C.; Kanitsoraphan, C.; Mekritthikrai, R.; Prasitlumkum, N.; Puttapiban, P.; Mekraksakit, P.; Vutthikraivit, W.; Sorajja, D. Wide QRS complex and the risk of major arrhythmic events in Brugada syndrome patients: A systematic review and meta-analysis. J. Arrhythmia 2019, 36, 143–152.
- Miles, C.; Asimaki, A.; Ster, I.C.; Papadakis, M.; Gray, B.; Westaby, J.; Finocchiaro, G.; Bueno-Beti, C.; Ensam, B.; Basu, J.; et al. Biventricular myocardial fibrosis and sudden death in patients with Brugada syndrome. J. Am. Coll. Cardiol. 2021, 78, 1511–1521.
- Zhang, Z.-H.; Barajas-Martínez, H.; Xia, H.; Li, B.; Capra, J.A.; Clatot, J.; Chen, G.-X.; Chen, X.; Yang, B.; Jiang, H.; et al. Distinct features of probands with early repolarization and Brugada syndromes carrying SCN5A pathogenic variants. J. Am. Coll. Cardiol. 2021, 78, 1603–1617.
- Tse, G.; Lee, S.; Liu, T.; Yuen, H.C.; Wong, I.C.K.; Mak, C.; Mok, N.S.; Wong, W.T. Identification of novel SCN5A single nucleotide variants in Brugada syndrome: A territory-wide study from Hong Kong. Front. Physiol. 2020, 11, 574590.
- Barc, J.; Tadros, R.; Glinge, C.; Chiang, D.Y.; Jouni, M.; Simonet, F.; Jurgens, S.J.; Baudic, M.; Nicastro, M.; Potet, F.; et al. Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility. Nat. Genet. 2022, 54, 232–239.
- Garcia-Elias, A.; Benito, B. Ion Channel Disorders and Sudden Cardiac Death. Int. J. Mol. Sci. 2018, 19, 692.
- Pranata, R.; Yonas, E.; Chintya, V.; Deka, H.; Raharjo, S.B. Association between PR Interval, First-degree atrioventricular block and major arrhythmic events in patients with Brugada syndrome—Systematic review and meta-analysis. J. Arrhythmia 2019, 35, 584–590.
- Saha, S.A.; Krishnan, K.; Madias, C.; Trohman, R.G. Combined right ventricular outflow tract epicardial and endocardial late potential ablation for treatment of Brugada storm: A case report and review of the literature. Cardiol. Ther. 2016, 5, 229–243.
- Di Domenico, M.; Cuda, G.; Scumaci, D.; Grasso, S.; Gaspari, M.; Curcio, A.; Oliva, A.; Ausania, F.; Di Nunzio, C.; Ricciardi, C.; et al. Biomarker discovery by plasma proteomics in familial Brugada Syndrome. Front. Biosci. 2013, 18, 564–571.
- Roterberg, G.; El-Battrawy, I.; Veith, M.; Liebe, V.; Ansari, U.; Lang, S.; Zhou, X.; Akin, I.; Borggrefe, M. Arrhythmic events in Brugada syndrome patients induced by fever. Ann. Noninvasive Electrocardiol. 2019, 25, 135–140.
- Zumhagen, S.; Zeidler, E.M.; Stallmeyer, B.; Ernsting, M.; Eckardt, L.; Schulze-Bahr, E. Tpeak–Tendinterval and Tpeak–Tend/QT ratio in patients with Brugada syndrome. Europace 2016, 18, 1866–1872.
- Tse, G.; Gong, M.; Li, C.K.H.; Leung, K.S.K.; Georgopoulos, S.; Bazoukis, G.; Letsas, K.P.; Sawant, A.C.; Mugnai, G.; Wong, M.C.S.; et al. T peak -T end, T peak -T end /QT ratio and T peak -T end dispersion for risk stratification in Brugada syndrome: A systematic review and meta-analysis. J. Arrhythmia 2018, 34, 587–597.
- Tse, G.; Lee, S.; Li, A.; Chang, D.; Li, G.; Zhou, J.; Liu, T.; Zhang, Q. Automated electrocardiogram analysis identifies novel predictors of ventricular arrhythmias in Brugada syndrome. Front. Cardiovasc. Med. 2021, 7, 399.
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC). Endorsed by: Association for European paediatric and congenital cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867.
- Pappone, C.; Santinelli, V. Brugada syndrome: Progress in diagnosis and management. Arrhythmia Electrophysiol. Rev. 2019, 8, 13–18.
- Antzelevitch, C.; Yan, G.-X.; Ackerman, M.J.; Borggrefe, M.; Corrado, D.; Guo, J.; Gussak, I.; Hasdemir, C.; Horie, M.; Huikuri, H.; et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Hear. Rhythm 2016, 13, e295–e324.
- Matusik, P.S.; Bryll, A.; Matusik, P.T.; Pac, A.; Popiela, T.J. Electrocardiography and cardiac magnetic resonance imaging in the detection of left ventricular hypertrophy: The impact of indexing methods. Kardiol. Pol. 2020, 78, 889–898.
- Kucharz, A.; Kułakowski, P. Fragmented QRS and arrhythmic events in patients with implantable cardioverter-defibrillators. Kardiol. Pol. 2020, 78, 1107–1114.
- Matusik, P.T. Biomarkers and cardiovascular risk stratification. Eur. Heart J. 2019, 40, 1483–1485.
- Matusik, P.T.; Małecka, B.; Lelakowski, J.; Undas, A. Association of NT-proBNP and GDF-15 with markers of a prothrombotic state in patients with atrial fibrillation off anticoagulation. Clin. Res. Cardiol. 2020, 109, 426–434.
- Asvestas, D.; Tse, G.; Baranchuk, A.; Bazoukis, G.; Liu, T.; Saplaouras, A.; Korantzopoulos, P.; Goga, C.; Efremidis, M.; Sideris, A.; et al. High risk electrocardiographic markers in Brugada syndrome. Int. J. Cardiol. Heart Vasc. 2018, 18, 58–64.
- Okólska, M.; Łach, J.; Matusik, P.T.; Pająk, J.; Mroczek, T.; Podolec, P.; Tomkiewicz-Pająk, L. Heart Rate variability and its associations with organ complications in adults after fontan operation. J. Clin. Med. 2021, 10, 4492.
- Pruszczyk, P.; Skowrońska, M.; Ciurzyński, M.; Kurnicka, K.; Lankei, M.; Konstantinides, S. Assessment of pulmonary embolism severity and the risk of early death. Pol. Arch. Intern. Med. 2021, 131.
- Letsas, K.P.; Vlachos, K.; Conte, G.; Efremidis, M.; Nakashima, T.; Duchateau, J.; Bazoukis, G.; Frontera, A.; Mililis, P.; Tse, G.; et al. Right ventricular outflow tract electroanatomical abnormalities in asymptomatic and high-risk symptomatic patients with Brugada syndrome: Evidence for a new risk stratification tool? J. Cardiovasc. Electrophysiol. 2021, 32, 2997–3007.
- Lee, S.; Zhou, J.; Li, K.H.C.; Leung, K.S.K.; Lakhani, I.; Liu, T.; Wong, I.C.K.; Mok, N.S.; Mak, C.; Jeevaratnam, K.; et al. Territory-wide cohort study of Brugada syndrome in Hong Kong: Predictors of long-term outcomes using random survival forests and non-negative matrix factorisation. Open Heart 2021, 8, e001505.
- Lee, S.; Wong, W.T.; Wong, I.C.K.; Mak, C.; Mok, N.S.; Liu, T.; Tse, G. Ventricular Tachyarrhythmia Risk in Paediatric/Young vs. Adult Brugada Syndrome Patients: A Territory-Wide Study. Front. Cardiovasc. Med. 2021, 8, 671666.
- Tse, G.; Zhou, J.; Lee, S.; Liu, T.; Bazoukis, G.; Mililis, P.; Wong, I.C.K.; Chen, C.; Xia, Y.; Kamakura, T.; et al. Incorporating latent variables using nonnegative matrix factorization improves risk stratification in Brugada syndrome. J. Am. Heart Assoc. 2020, 9, e012714.
- Letsas, K.P.; Asvestas, D.; Baranchuk, A.; Liu, T.; Georgopoulos, S.; Efremidis, M.; Korantzopoulos, P.; Bazoukis, G.; Tse, G.; Sideris, A.; et al. Prognosis, risk stratification, and management of asymptomatic individuals with Brugada syndrome: A systematic review. Pacing Clin. Electrophysiol. 2017, 40, 1332–1345.
- Marsman, E.M.J.; Postema, P.G.; Remme, C.A. Brugada syndrome: Update and future perspectives. Heart 2021, 2020, 318258.
- Honarbakhsh, S.; Providência, R.; Lambiase, P.D.; Centre, S.B.H.B.H. Risk stratification in Brugada syndrome: Current status and emerging approaches. Arrhythmia Electrophysiol. Rev. 2018, 7, 79–83.
- Bernardo, M.; Tiyyagura, S.R. A case of type I and II Brugada phenocopy unmasked in a patient with normal baseline electrocardiogram (ECG). Am. J. Case Rep. 2018, 19, 21–24.
- Matusik, P.T.; Komar, M.; Podolec, J.; Karkowski, G.; Lelakowski, J.; Podolec, P. Exercise ECG unmasked Brugada sign: Manifestation of the risk of sports-associated sudden cardiac arrest (RCD code: V-1A.1). J. Rare Cardiovasc. Dis. 2017, 3, 92–97.
- Abe, A.; Kobayashi, K.; Yuzawa, H.; Sato, H.; Fukunaga, S.; Fujino, T.; Okano, Y.; Yamazaki, J.; Miwa, Y.; Yoshino, H.; et al. Comparison of late potentials for 24 hours between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy using a novel signal-averaging system based on holter ECG. Circ. Arrhythmia Electrophysiol. 2012, 5, 789–795.
- Kawada, S.; Morita, H.; Antzelevitch, C.; Morimoto, Y.; Nakagawa, K.; Watanabe, A.; Nishii, N.; Nakamura, K.; Ito, H. Shanghai Score system for diagnosis of brugada syndrome: Validation of the score system and system and reclassification of the patients. JACC Clin. Electrophysiol. 2018, 4, 724–730.
- Wilde, A.A.M. The Shanghai score system in Brugada syndrome: Using it beyond a diagnostic score. JACC Clin. Electrophysiol. 2018, 4, 731–732.
- Wijeyeratne, Y.D.; Tanck, M.W.; Mizusawa, Y.; Batchvarov, V.; Barc, J.; Crotti, L.; Bos, J.M.; Tester, D.J.; Muir, A.; Veltmann, C.; et al. SCN5A Mutation Type and a Genetic Risk Score Associate Variably with Brugada Syndrome Phenotype in SCN5A Families. Circ. Genom. Precis. Med. 2020, 13, e002911.
- Veltmann, C.; Papavassiliu, T.; Konrad, T.; Doesch, C.; Kuschyk, J.; Streitner, F.; Haghi, D.; Michaely, H.; Schoenberg, S.; Borggrefe, M.; et al. Insights into the location of type I ECG in patients with Brugada syndrome: Correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm 2012, 9, 414–421.
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American college of cardiology/American Heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2018, 72, e91–e220.
- Wakamiya, A.; Kamakura, T.; Shinohara, T.; Yodogawa, K.; Murakoshi, N.; Morita, H.; Takahashi, N.; Inden, Y.; Shimizu, W.; Nogami, A.; et al. Improved risk stratification of patients with Brugada syndrome by the new Japanese circulation society guideline―A multicenter validation study. Circ. J. 2020, 84, 2158–2165.
- Priori, S.G.; Gasparini, M.; Napolitano, C.; Della Bella, P.; Ottonelli, A.G.; Sassone, B.; Giordano, U.; Pappone, C.; Mascioli, G.; Rossetti, G.; et al. Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 2012, 59, 37–45.
- Bigi, M.A.B.; Aslani, A.; Shahrzad, S. aVR sign as a risk factor for life-threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm 2007, 4, 1009–1012.
- Tsai, C.-F.; Chuang, Y.-T.; Huang, J.-Y.; Ueng, K.-C. Long-term prognosis of febrile individuals with right precordial coved-type ST-segment elevation Brugada pattern: A 10-year prospective follow-up study. J. Clin. Med. 2021, 10, 4997.
- Iacopino, S.; Chierchia, G.-B.; Sorrenti, P.; Pesce, F.; Colella, J.; Fabiano, G.; Campagna, G.; Petretta, A.; Placentino, F.; Filannino, P.; et al. dST-Tiso Interval, a novel electrocardiographic marker of ventricular arrhythmia inducibility in individuals with ajmaline-induced Brugada type I pattern. Am. J. Cardiol. 2021, 159, 94–99.
- Ishikawa, T.; Kimoto, H.; Mishima, H.; Yamagata, K.; Ogata, S.; Aizawa, Y.; Hayashi, K.; Morita, H.; Nakajima, T.; Nakano, Y.; et al. Functionally validated SCN5A variants allow interpretation of pathogenicity and prediction of lethal events in Brugada syndrome. Eur. Heart J. 2021, 42, 2854–2863.
- Letsas, K.P.; Vlachos, K.; Efremidis, M.; Dragasis, S.; Korantzopoulos, P.; Tse, G.; Liu, T.; Bazoukis, G.; Niarchou, P.; Prappa, E.; et al. Right ventricular outflow tract endocardial unipolar substrate mapping: Implications in risk stratification of Brugada syndrome. Rev. Cardiovasc. Med. 2022, 23, 044.
- Chung, C.T.; Bazoukis, G.; Radford, D.; Coakley-Youngs, E.; Rajan, R.; Matusik, P.T.; Liu, T.; Letsas, K.P.; Lee, S.; Tse, G. Predictive risk models for forecasting arrhythmic outcomes in Brugada syndrome: A focused review. J. Electrocardiol. 2022, 72, 28–34.
- Probst, V.; Goronflot, T.; Anys, S.; Tixier, R.; Briand, J.; Berthome, P.; Geoffroy, O.; Clementy, N.; Mansourati, J.; Jesel, L.; et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur. Heart J. 2021, 42, 1687–1695.
- Honarbakhsh, S.; Providencia, R.; Garcia-Hernandez, J.; Martin, C.A.; Hunter, R.J.; Lim, W.Y.; Kirkby, C.; Graham, A.J.; Sharifzadehgan, A.; Waldmann, V.; et al. A primary prevention clinical risk score model for patients with Brugada syndrome (BRUGADA-RISK). JACC: Clin. Electrophysiol. 2021, 7, 210–222.
- Letsas, K.P.; Georgopoulos, S.; Vlachos, K. Brugada syndrome:risk stratification and management corresponding author. J. Atr. Fibrillation 2016, 7, 79–83.
- Dereci, A.; Yap, S.C.; Schinkel, A.F.L. Meta-Analysis of clinical outcome after implantable cardioverter-defibrillator implantation in patients with Brugada syndrome. JACC Clin. Electrophysiol. 2019, 5, 141–148.
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Nasi, G.; Rivera, M.; Mitrani, R.D.; Goldberger, J.J. Ablation strategies for the management of symptomatic Brugada syndrome: A systematic review. Heart Rhythm 2018, 15, 1140–1147.
- Nademanee, K.; Veerakul, G.; Chandanamattha, P.; Chaothawee, L.; Ariyachaipanich, A.; Jirasirirojanakorn, K.; Likittanasombat, K.; Bhuripanyo, K.; Ngarmukos, T. Arrhythmia/electrophysiology prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011, 123, 1270–1279.
- Asada, S.; Morita, H.; Watanabe, A.; Nakagawa, K.; Nagase, S.; Miyamoto, M.; Morimoto, Y.; Kawada, S.; Nishii, N.; Ito, H. Indication and prognostic significance of programmed ventricular stimulation in asymptomatic patients with Brugada syndrome. Europace 2020, 22, 972–979.




