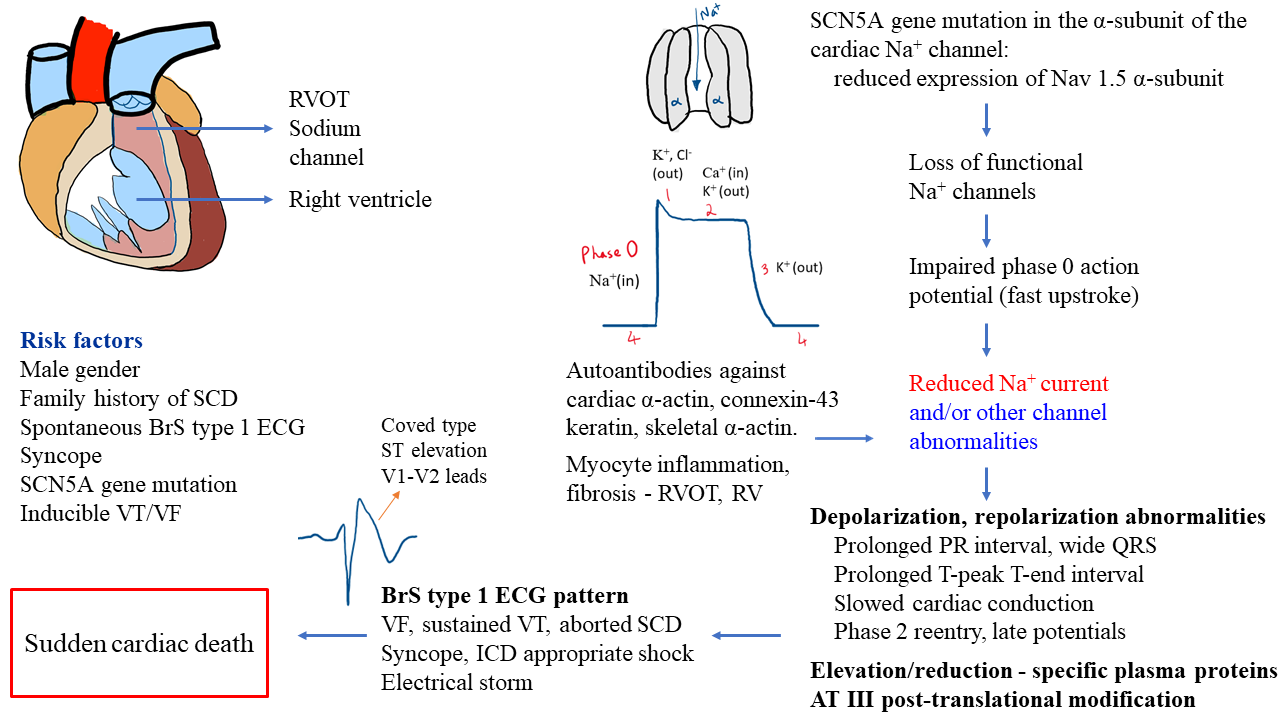
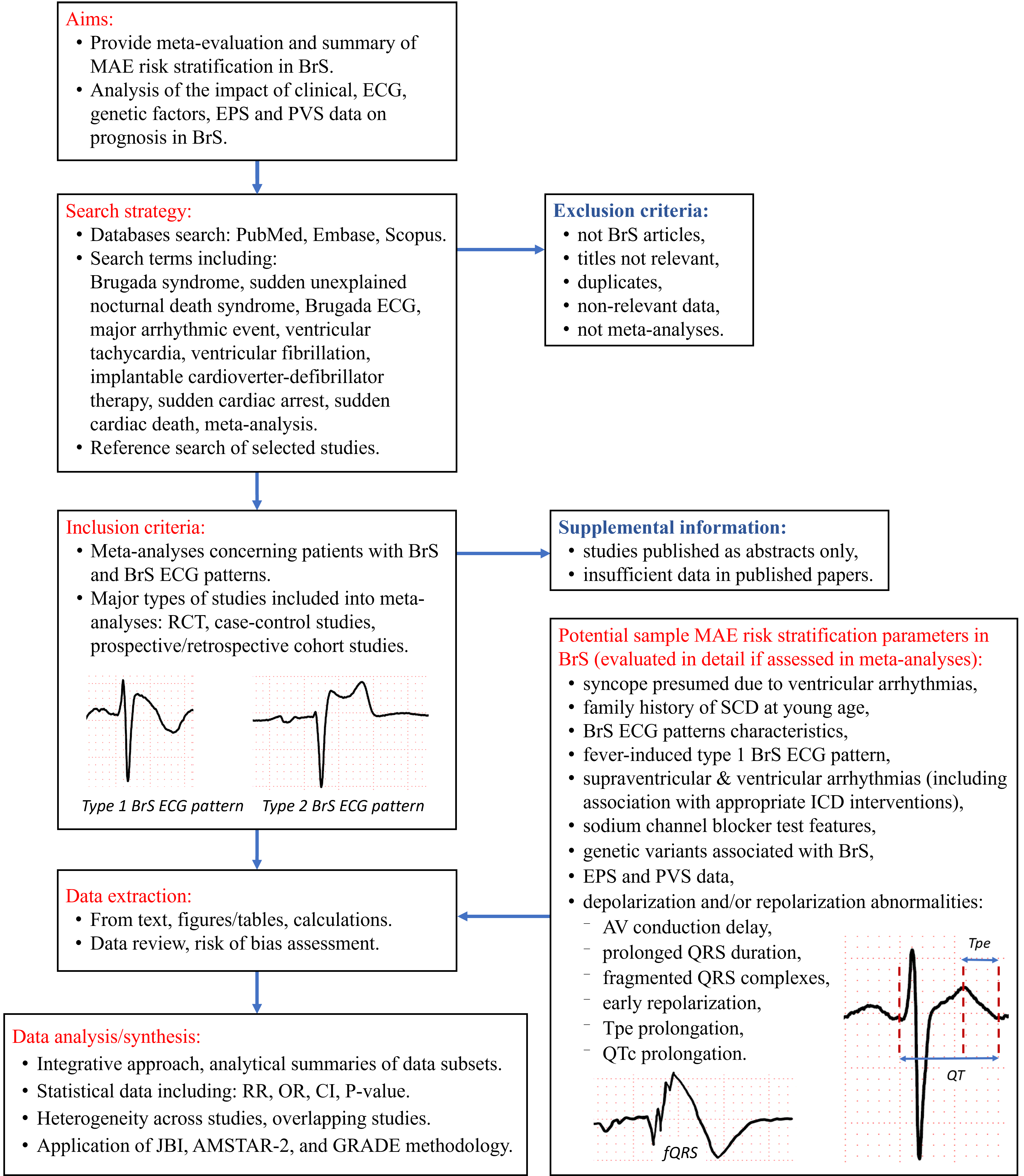
The definitions of BrS can vary depending on the guidelines used. The European Society of Cardiology (ESC) guidelines proposes that any subject with spontaneous or drug-induced type 1 Brugada ECG pattern be classified as BrS [
28]. However, some investigators suggest that this may lead to overdiagnosis, and specific symptoms or clinical data are required to confirm diagnosis [
29,
30]. Clinical, ECG, and laboratory markers have been found to be useful in diagnostics and risk stratification in diverse groups of patients [
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41]. Despite progress in SCD prevention, the optimal diagnostics and risk stratification in BrS are a major clinical challenge [
42,
43,
44].
2.1. Diagnostics in BrS
The ESC definition of BrS diagnosis states that patients must display BrS ECG pattern with ST-segment elevation ≥ 2 mm in at least one lead (V1-V2) placed in the second, third, or fourth intercostal spaces [
28]. This may appear spontaneously or following intravenous drug provocation with class Ia (ajmaline or procainamide) or class Ic (flecainide or pilsicainide) sodium channel blockers [
28]. The BrS type 1 ECG pattern may also be induced by fever and exercise tests [
8,
9,
10,
11,
45]. The unique ECG pattern is often short-lasting, and only depending on standard 12-lead ECG may lead to underdiagnosis of 65% patients, especially those who need modified high leads or drug provocation [
9]. Therefore, prolonged cardiac monitoring might be highly valuable for diagnostic process [
46]. When the Brugada ECG pattern is present without life-threatening arrhythmias or SCD, after exclusion of BrS, it is known as Brugada phenocopy [
8].
The Shanghai scoring system for BrS diagnosis is based on ECG, family history, clinical symptoms, and genetics, and assigns a score of ≥3.5 for probable and/or definitive BrS (type 1 BrS ECG pattern–spontaneous or drug-induced), a score from 2 to 3 for possible BrS, and a score of <2 is nondiagnostic [
9,
47,
48]. Additionally, a score of 3 was for fever-induced BrS type 1 ECG, a score of 2 for convertible drug-induced type 2 or 3 BrS ECG pattern, a score of 2 for definite BrS in family (first-/second-degree relative), a score of 0.5 for atrial fibrillation (AF) or atrial flutter in patients younger than age 30 ( no alternative etiology), and a score of 0.5 for probable pathologic genetic mutation which may lead to BrS [
9]. SCN5A gene-mutation type and a BrS genetic risk score is associated with BrS phenotype in patients with spontaneous type 1 BrS ECG pattern or family members with SCN5A mutations [
49]. Importantly, MRI studies have shown a correlation between maximal ECG ST segment elevation and maximal RVOT area in the presence of BrS type 1 ECG pattern [
50].
2.2. Risk Stratification in BrS
ESC guidelines on ventricular arrhythmias and the prevention of SCD [
28] recommend focusing on risk stratification and clinical decisions in the presence of previous SCA or documented spontaneous sustained VT, spontaneous diagnostic type 1 BrS ECG pattern, syncopal episodes, and inducible VF during programmed ventricular stimulation (PVS) (using two–three extrastimuli at two sites). According to the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society (AHA/ACC/HRS) recommendations, for additional risk stratification in asymptomatic BrS patients and in patients with spontaneous type 1 BrS ECG pattern, electrophysiological study (EPS) with PVS (using single and double extrastimuli) may be beneficial [
51]. Wakamiya et al. evaluated the emphasis of arrhythmic syncope history or unexplained syncope and VF inducibility by ≤two extrastimuli during PVS according to new guidelines of the Japanese Circulation Society to help determine ICD indication in patients with BrS [
52]. The research studied 234 BrS patients where 20% had VF history, 43% had syncope history, and 37% were asymptomatic at diagnosis [
52]. Patients underwent PVS at RV apex or RVOT (1–3 extrastimuli) and mean follow-up was 6.9 ± 5.2 years [
52]. The study underlined a less aggressive approach for PVS in BrS risk stratification.
Spontaneous type 1 BrS ECG pattern and syncopal episode history, fragmented QRS (fQRS), and ventricular effective refractory period (VRP) <200 milliseconds have been independently associated with ventricular arrhythmic events in BrS [
53]. Moreover, prominent R wave (≥0.3 mV or R/q ≥ 0.75) in lead aVR (aVR sign) was identified to be associated with arrhythmic events in BrS [
54]. Fever may precipitate both Brugada type 1 and 2 ECG patterns in patients who have normal baseline ECG [
8], and predispose to the development of life-threatening ventricular arrhythmias and SCD [
10]. However, a recent study indicated that asymptomatic patients with fever-induced type 1 BrS ECG pattern, negative family history of sudden death, and without spontaneous type 1 BrS ECG pattern are at low risk for future arrhythmic events [
55].
Prolonged QRS complex duration, >120 milliseconds on standard 12-lead ECG due to slowed depolarization, was more pronounced in BrS patients expressing symptoms and may predict future MAE [
12]. Moreover, T-peak to T-end (Tpe) intervals were identified as novel ECG markers in BrS patients for MAE prediction. High-risk BrS patients had longer Tpe interval in lead V1 and Tpeak-Tend/QT ratio compared to low-risk BrS patients [
25]. Recently, based on 12-lead ECG data extracted from automated measurements in BrS patients, novel markers (i.e., ST slope) in predicting arrhythmic events in BrS were identified [
27]. The authors stated that, using a weighted scoring system determined from QRS frontal axis, QRS duration, S wave duration and ST slope in lead I, as well as R wave duration in lead III, spontaneous VT/VF incidence may be predicted [
27].
A new research performed on patients with drug-induced type 1 BrS ECG pattern who underwent PVS showed that a novel ECG marker dST-Tiso interval (the longest interval from V1-2 in the second, third, or fourth intercostal space) following ajmaline injection to be a significant predictor for the inducibility of ventricular arrhythmias (sustained or hemodynamically significant polymorphic VT of VF requiring direct current shock) [
56]. The dST-Tiso interval lies in between the initiation and termination (at the isoelectric line) of the elevated coved ST-segment [
56]. The dST-Tiso interval displayed adjusted OR 1.03 (95% CI: 1.01–1.04,
p < 0.001) for ventricular arrhythmias inducibility. At the same time, dST-Tiso interval > 300 ms displayed 92.0% sensitivity, 90.2% specificity, 82.1% positive predictive value, and 95.8% negative predictive value for VT/VF inducibility prediction [
56].
SCN5A gene variants may be important predictors of fatal events in BrS and valuable in risk stratification [
57]. Loss-of-function SCN5A mutations have shown association with prolonged ECG conduction parameters (P wave or QRS durations) and increased occurrence of lethal arrhythmic events compared to the non-loss-of-function SCN5A mutations or SCN5A(−) BrS patients [
57].
In BrS individuals, the presence of structural anomalies in the epicardium of the RVOT may contribute to arrhythmias [
38]. Endocardial unipolar electroanatomical mapping technology may identify RVOT electrical abnormalities with VF inducibility during PVS and assist in BrS risk stratification [
38,
58]. Endocardial high-density electroanatomical mapping may permit BrS risk stratification in asymptomatic patients (referred for PVS) [
38].
Published studies have discussed clinical risk score models in patients with BrS and were reviewed recently in detail [
59]. Briefly, the Shanghai Brugada scoring system was predictive for malignant arrhythmic events among patients evaluated for BrS who were asymptomatic (
n = 271), experienced syncopal episodes (
n = 99), or had previous VF (
n = 23) [
47]. Importantly, there were no malignant arrhythmic events in patients with a score of 3 or less (possible or nondiagnostic BrS) [
47]. In a multicentric study of 1613 BrS patients, 20% symptomatic (after aborted SCA or syncope) at diagnosis, researchers evaluated the Shanghai score of all patients and Sieira score of 461 patients (mean follow-up 6.5 ± 4.7 years) [
60]. While both scoring systems identified arrhythmic events risk in patients with significantly elevated or reduced scores, risk stratification was challenging in intermediate-risk patients, e.g., Sieira score 2–4 [
60]. Another multicenter international study of 1110 BrS patients developed a risk-score model for SCD or ventricular arrhythmias, and studied 16 proposed ECG/clinical markers for risk stratification and ICD therapy indication [
61]. In a median follow-up of 5.33 years, 10.3% of patients had SCD or ventricular arrhythmias, and increased risk was associated with four factors: spontaneous type-1 BrS ECG pattern (14 points), possible arrhythmic syncope or early repolarization in peripheral leads (each 12 points), and type-1 BrS ECG pattern in peripheral leads (9 points) [
61].