
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Philipp E Scherer | -- | 2142 | 2022-04-15 14:48:26 | | | |
| 2 | Rita Xu | Meta information modification | 2142 | 2022-04-18 04:44:31 | | |
Video Upload Options
Adipocytes from the superficial layer of subcutaneous adipose tissue undergo cyclic de- and re-differentiation, which can significantly influence the development of skin inflammation under different cutaneous conditions. This inflammation can be connected with local loading of the reticular dermis with lipids released due to de-differentiation of adipocytes during the catagen phase of the hair follicle cycle. Alternatively, the inflammation parallels a widespread release of cathelicidin, which typically takes place in the anagen phase (especially in the presence of pathogens). Additionally, trans-differentiation of dermal adipocytes into myofibroblasts, which can occur under some pathological conditions, can be responsible for the development of collateral scarring in acne.
1. Introduction
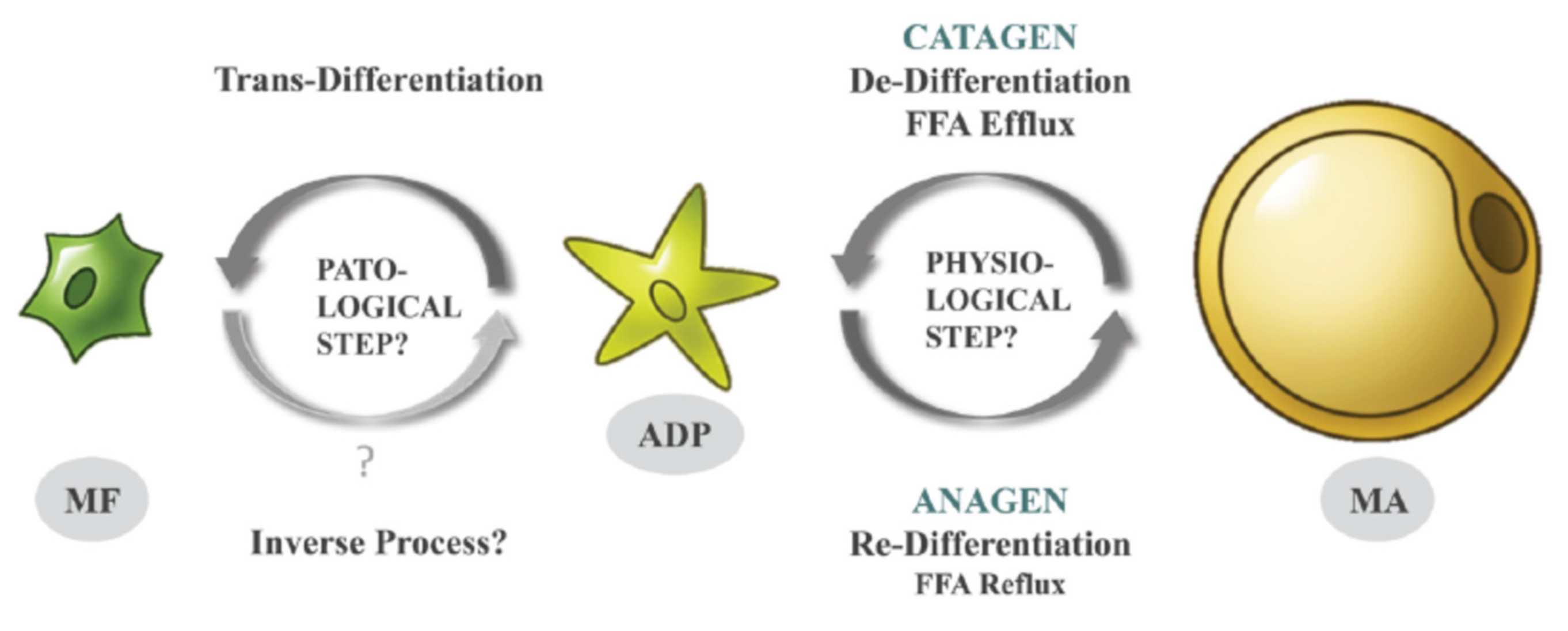
2. DWAT Layer in Human Skin
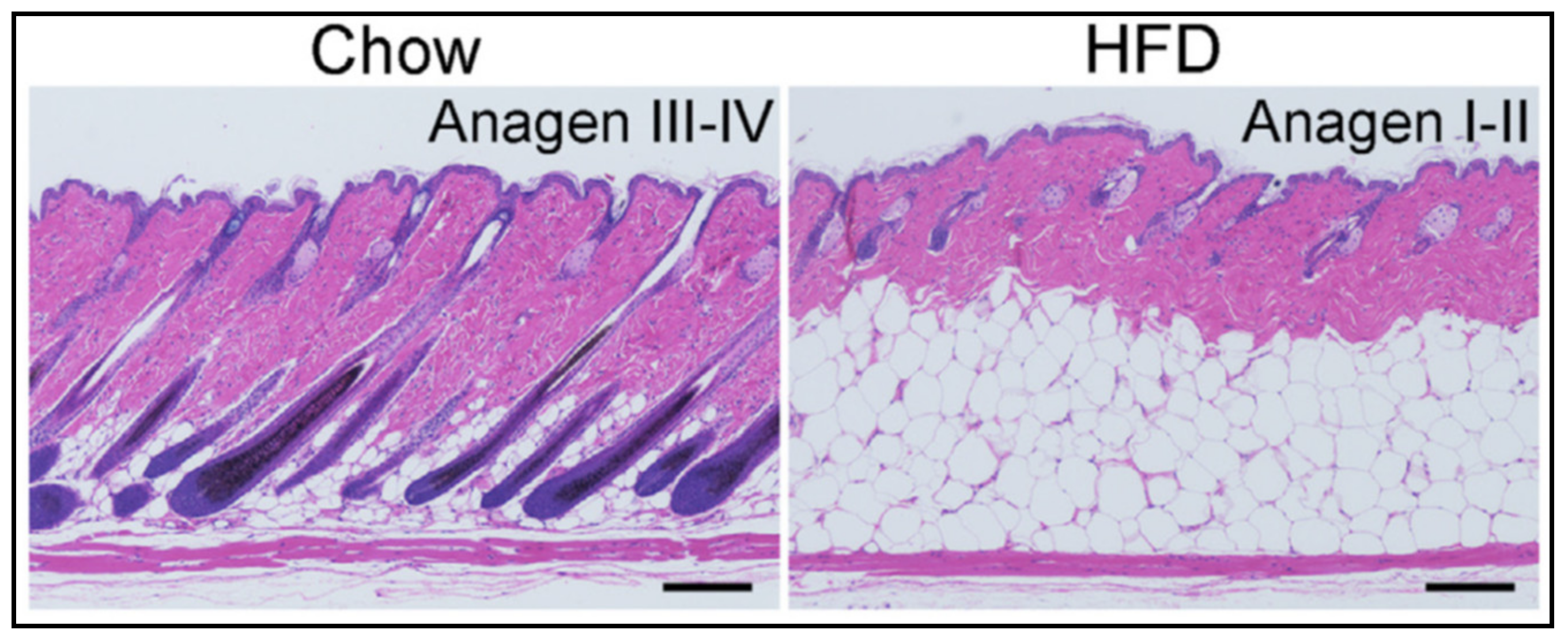
3. Connection of Acne and Psoriasis to the HF Cycle and High Fat Diet
3.1. Acne vulgaris
3.2. Psoriasis vulgaris
References
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes: From irrelevance to metabolic targets? Trends Endocrinol. Metab. 2016, 27, 1–10.
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163–173.
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. The Role of Immature and Mature Adipocytes in Hair Cycling. Trends Endocrinol. Metab. 2019, 30, 93–105.
- Zhang, Z.; Shao, M.; Hepler, C.; Zi, Z.; Zhao, S.; An, Y.A.; Zhu, Y.; Ghaben, A.L.; Wang, M.Y.; Li, N.; et al. Dermal adipose tissue has high plasticity and undergoes reversible de-differentiation in mice. J. Clin. Invest. 2019, 129, 5327–5342.
- Kruglikov, I.L.; Scherer, P.E. Caveolin-1 expression as an etiopathogenic factor in acne. Exp. Dermatol. 2019, 29, 177–183.
- Zhang, Z.; Kruglikov, I.L.; Zhao, S.; Zi, Z.; Gliniak, C.M.; Li, N.; Wang, M.Y.; Zhu, Q.; Kusminski, C.M.; Scherer, P.E. Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp. Dermatol. 2021, 30, 102–111.
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. Skin aging: Dermal adipocytes metabolically reprogram dermal fibroblasts. BioEssays 2022, 44, 2100207.
- Zhang, L.-J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71.
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; Hsi, T.C.; et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017, 355, 748–752.
- Chen, S.X.; Zhang, L.-J.; Gallo, R.L. Dermal White Adipose Tissue: A Newly Recognized Layer of Skin Innate Defense. J. Investig. Dermatol. 2019, 139, 1002–1009.
- Kruglikov, I.; Trujillo, O.; Kristen, Q.; Isac, K.; Zorko, J.; Fam, M.; Okonkwo, K.; Mian, A.; Thanh, H.; Koban, K.; et al. The Facial Adipose Tissue: A Revision. Facial Plast. Surg. 2016, 32, 671–682.
- Driskell, R.R.; Jahoda, C.A.B.; Chuong, C.-M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631.
- Kasza, I.; Kühn, J.-P.; Völzke, H.; Hernando, D.; Xu, Y.G.; Siebert, J.W.; Gibson, A.L.F.; Yen, C.L.E.; Nelson, D.W.; MacDougald, O.A.; et al. Contrasting recruitment of skin-associated adipose depots during cold challenge of mouse and human. J. Physiol. 2021, 600, 847–868.
- Kasza, I.; Hernando, D.; Roldán-Alzate, A.; Alexander, C.M.; Reeder, S.B. Thermogenic profiling using magnetic resonance imaging of dermal and other adipose tissues. JCI Insight 2016, 1, e87146.
- Azzi, L.; El-Alfy, M.; Martel, C.; Labrie, F. Gender Differences in Mouse Skin Morphology and Specific Effects of Sex Steroids and Dehydroepiandrosterone. J. Investig. Dermatol. 2005, 124, 22–27.
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029.
- Jeremy, A.H.; Holland, D.B.; Roberts, S.G.; Thomson, K.F.; Cunliffe, W.J. Inflammatory Events Are Involved in Acne Lesion Initiation. J. Investig. Dermatol. 2003, 121, 20–27.
- Snast, I.; Dalal, A.; Twig, G.; Astman, N.; Kedem, R.; Levin, D.; Erlich, Y.; Leshem, Y.A.; Lapidoth, M.; Hodak, E.; et al. Acne and obesity: A nationwide study of 600,404 adolescents. J. Am. Acad. Dermatol. 2019, 81, 723–729.
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40.
- Van Scott, E.J.; MacCardle, R.C. Keratinization of the Duct of the Sebaceous Gland and Growth Cycle of the Hair Follicle in the Histogenesis of Acne in Human Skin1. J. Investig. Dermatol. 1956, 27, 405–429.
- Paus, R.; Link, R.E. The psoriatic epidermal lesion and anagen hair growth may share the same “switch-on” mechanism. Yale J. Biol. Med. 1988, 61, 467–476.
- Kasumagić-Halilović, E.; Prohić, A.; Begović, B. TrichoScan as a method to determine hair root pattern in patients with scalp psoriasis. Acta Dermatovenerol. Croat. 2010, 18, 146–150.
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes and hair cycling: Is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Dermatol. 2016, 25, 258–262.
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2013, 68, 654–662.
- Setty, A.R.; Curhan, G.; Choi, H.K. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Int. Med. 2007, 167, 1670–1675.
- Kumar, S.; Curhan, G.; Li, T.; Qureshi, A.A. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J. Eur. Acad. Dermatol. Venereol. 2012, 27, 1293–1298.
- Alotaibi, H.A. Effects of Weight Loss on Psoriasis: A Review of Clinical Trials. Cureus 2018, 10, e3491.
- Shibata, S.; Tada, Y.; Asano, Y.; Hau, C.S.; Kato, T.; Saeki, H.; Yamauchi, T.; Kubota, N.; Kadowaki, T.; Sato, S. Adiponectin Regulates Cutaneous Wound Healing by Promoting Keratinocyte Proliferation and Migration via the ERK Signaling Pathway. J. Immunol. 2012, 189, 3231–3241.
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015, 6, 7687.
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009.
- Kruglikov, I.L.; Scherer, P.E.; Wollina, U. Are dermal adipocytes involved in psoriasis? Exp. Dermatol. 2016, 25, 812–813.
- Kruglikov, I.L.; Wollina, U. Local effects of adipose tissue in psoriasis and psoriatic arthritis. Psoriasis 2017, 7, 17–25.
- Shirsath, N.; Mayer, G.; Singh, T.P.; Wolf, P. 8-methoxypsoralen plus UVA (PUVA) therapy normalizes signalling of phos-phorylated component of mTOR pathway in psoriatic skin of K5.hTGFβ1 transgenic mice. Exp. Dermatol. 2015, 24, 889–891.
- Dattola, A.; Altobelli, S.; Marsico, S.; Plastina, D.; Nistico, S.P.; Cavallo, A.; Floris, R.; Bianchi, L.; Guazzaroni, M. Hypodermal adipose tissue sonoelastography for monitoring treatment response in patients with plaque psoriasis. Photomed Laser Surg. 2017, 35, 484–491.
- Kruglikov, I.L.; Wollina, U. The role of subcutaneous adipose tissue in psoriasis. J. Biol. Regul. Homeost. Agents 2018, 32, 159–161.




