
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksander Ejsmont | -- | 3513 | 2022-04-13 14:36:36 | | | |
| 2 | Lindsay Dong | + 4 word(s) | 3517 | 2022-04-15 05:14:14 | | | | |
| 3 | Lindsay Dong | Meta information modification | 3517 | 2022-04-18 02:35:21 | | |
Video Upload Options
Nowadays, materials with great potential for environmental protection are being sought. Metal–organic frameworks, in particular those with cobalt species as active sites, have drawn considerable interest due to their excellent properties. With the use of Co-based metal–organic frameworks (MOFs) as photocatalysts in reactions (dye degradation, water oxidation and splitting, carbon dioxide reduction, in addition to the oxidation of organic compounds), even over 90% degradation efficiencies of various dyes (e.g., methylene blue) can be achieved.
1. Introduction
2. Photocatalytic Degradation of Dyes
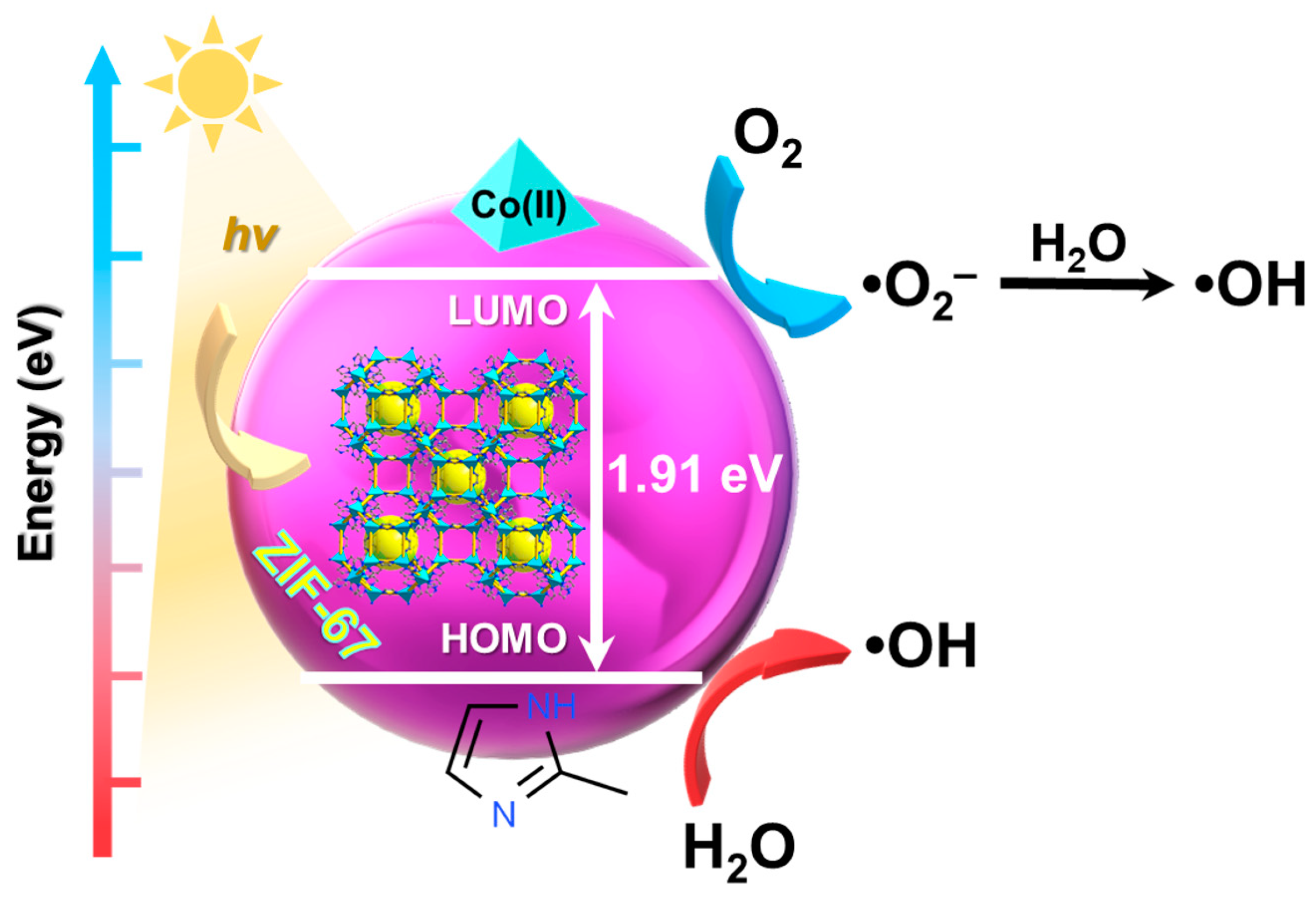
Textile, paper, and clothing industries are the main cause of the existence of organic dyes in water, which contributes to significant environmental pollution. Industrial effluents that contain toxic and non-biodegradable colorants are highly dangerous toward living organisms, thus their removal from water reservoirs is necessary. Over the past few years, photodegradation has become popular since it carries numerous advantages. The reaction may be conducted at room temperature and lasts only a few hours. Moreover, contaminants can be mineralized to harmless molecules (e.g., water, carbon dioxide) through the in situ generation of radicals without forming harmful secondary products. Light intensity, pH, or adsorption properties can affect the photodegradation of dyes. With the increase in the irradiation intensity, reactive oxygen species are generated at a higher rate and the photocatalytic performance is improved. Electrostatic interactions between the reactants are determined by the pH value, however, each catalyst may work more efficiently at a different pH. Although the moderate dye adsorption enhances the degradation yield, after exceeding a certain limit it can be unfavourable. Not only do fewer photons reach surface active sites, but also the molecules of a dye can act as sensitizers that absorb electrons, scattering them in unwanted directions.
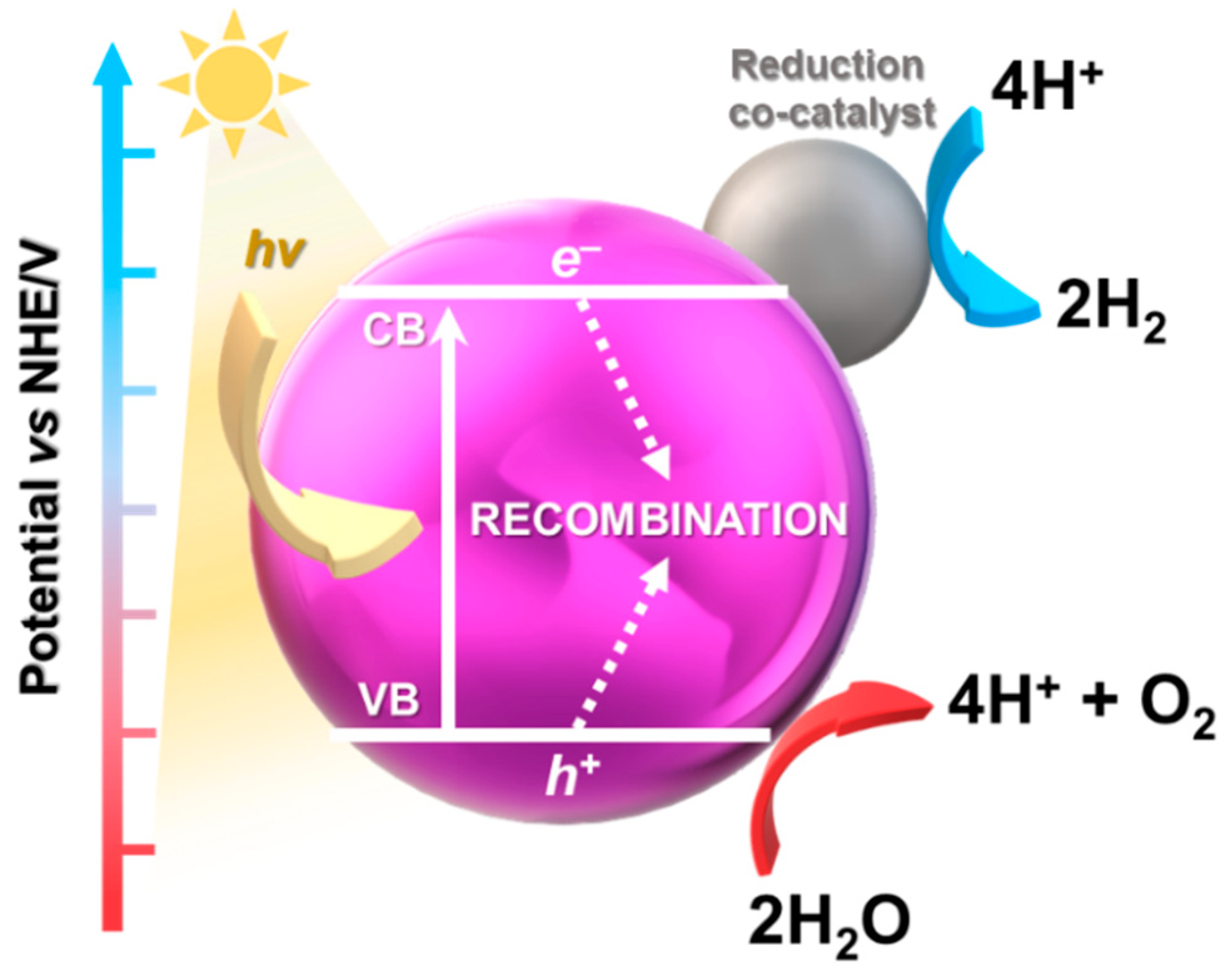
In the first step of the photocatalytic process, pollutants are transported from the environment to the surface of the photocatalyst where oxidation-reduction reactions take place. Photogenerated electrons in the CB and holes in the VB force these reactions to occur. Afterward, the products are desorbed and returned to the liquid phase. In order to generate electron-hole pairs, the energy of the photons must be equal to or exceed the band gap of the photocatalyst [32]. In the photodegradation of dyes, materials such as oxides (TiO2, ZnO), metal salts, or chalcogenides (e.g., CdS, Sb2S3, MnS), including their composites were described, although recently cobalt-based MOFs have been willingly utilized [33]. The large surface area, a wide selection of metallic centres and organic linkers, as well as the possibility to control morphological properties make MOFs excellent potential photocatalysts [33][34].
3. Photocatalytic Reactions with Water
3.1. Photocatalytic Water Oxidation
3.2. Photocatalytic Water Splitting
| Photocatalyst | TOF; TON; Generated Product Amount |
Ref. |
|---|---|---|
| Phodo-driven water oxidation | ||
| ZIF-67 | TOF = 0.035 s−11 | [24] |
| Co4-bdt | TOF = 3.050 s−1 | [54] |
| ([CoIICoIIIW11O39(H2O)]−7@MIL-101 | TOF = 0.48 s−1 | [55] |
| [Co4(PW9O34)2(H2O)2]−10@MIL-101 | TOF = 0.53 s−1 | [55] |
| Cox/MIL-101 | TOF = 0.012 s−1 | [56] |
| P2W18Co4@MOF-545 | TOF = 0.040 s−1 | [57] |
| P2W18Co4@MOF-545 film | TON = 1600 | [58] |
| ZIF-67 | 9.8 µmol of O2 | [59] |
| Co-MOF-74@ZIF-67 | 15.0 µmol of O2 | [59] |
| Co-MOF-74 | 11.8 µmol of O2 | [59] |
| CoP@CoOx | 901.5 mmol g−1 h−1 of O2 | [60] |
| Photoelectrocatalytic (PEC) water splitting for hydrogen generation | ||
| ZIF-67 film | 48.5 μmol g−1 of H2 | [61] |
| Cox/NH2-MIL-125(Ti) | TOF = 0.8 h−1 | [62] |
| Co3-XL | 23.05 µmol g−1 h−1 of H2 | [63] |
| (Co0.5[Ru(tpyCOO)2]PF6) | 589 μmol g−1 h−1 of H2 | [64] |
| Co4S3/CdS | 12 360 µmol g−1 h−1 of H2 | [65] |
4. Photocatalytic Carbon Dioxide Reduction
MOFs address many problems associated with photocatalytic processes due to their unique features. In the case of reaction with gases, MOFs show high potential in CO2 capture [66]. If the high adsorption capacity is properly coupled with the photoelectrocatalytic properties of MOFs, it can result in high-performance materials [67]. The general mechanism of CO2 reduction with MOFs proceeds in the following manner. In the beginning, light excites a photosensitizer, which then undergoes reductive quenching by a sacrificial electron donor. The reduced photosensitizer donates its electrons to the MOF with adsorbed CO2. At this stage, the CO2 is reduced to CO2*. Subsequently, the CO2* accepts protons generated from the decomposition of H2O and forms an COOH* intermediate to be further reduced to CO*. Then, CO* is desorbed, resulting in the formation of CO [68].
In the development of materials for CO2 reduction, systems capable of generating multielectrons are required [69]. For a MOF as a catalyst, the LUMO must be above the redox potential for the CO2 reduction half-reaction, which depends on the resulting product. For by far the most common CO2-to-CO conversion via Co-based MOF photocatalysts, the reduction potential is −0.53 V. By tuning the MOF composition, one can influence the optical and electronic responses i.e., HOMO and LUMO energy levels. In addition, the sorption capacity of the MOF is very important. Therefore, its textural parameters (e.g., surface area, pore volume) and improved affinity towards CO2 (e.g., by amine functionalities) must be attained in order to proceed efficient photoreduction [70]. For an effective CO2 conversion process, accessibility to the active sites is vital, thus 2D nanosheet MOFs with better exposed active sites are preferred. Defect engineering also contributes to improved access to nodes. Furthermore, defects play an important role in the excited state and charge relaxation pathways. Higher defect concentrations lead to longer excited-state lifetimes. This is attributed to the trapping of electrons in oxygen vacancies and holes in hydroxyl groups at the surface, which have been shown to play an important role in the CO2 reduction mechanism [71]. The conversion of CO2 can often lead to the formation of many products, but also to the generation of hydrogen from water. Therefore, it is quite challenging to design a material that has high selectivity for a single product without carrying out trial studies. The selectivity of Co-MOFs to receive one type of product in excess is still unclear. It requires a combination of theoretical calculations and the compilation of many parameters that represent a large system of variables during the process. In general, for any cobalt-based semiconductor, it is important to study the light intensity and photon energy, which must be applied to excite semiconductors. The photo-generated electrons and holes affect the kinetics of the reduction rate and ultimately the product selectivity. By attempting to select appropriate co-catalysts, it is possible to mitigate their effect on selectivity. Additionally, a key step is the desorption of the product from the material, which, if hindered, can result in low yields and poor selectivity. In photocatalytic CO2 conversion, it is necessary to couple a gas chromatography system equipped with a thermal conductivity detector (TCD), a hydrogen flame ionization detector (FID), and mass spectrometry to rigorously monitor the reaction products. By combining excitation features, catalyst and co-catalyst band structures, charge separation efficiency, and reactions on the material surface, it will be attainable to control product selectivity and material efficiency [72].
Cobalt-based complexes e.g., cobalt(II) tripodal or tetradentate ligand complexes, have been considered efficient catalysts of CO2 reduction with promising results in terms of high selectivity (95–100%) [73][74]. Most importantly, MOFs are characterized by high CO2 sorption capacities, which could be a key aspect for the implementation of these materials on a larger scale. One of the highest CO2 adsorption capacities of 288 mg g−1 was reported for Co-MOF-74 and attributed to its well-developed surface area of 1314 m2 g−1 [75]. Therefore, the photocatalytic activities of Co-MOFs were tested in CO2 reduction process. For example, Co-based MOFs [Co2(μ-Cl)2(bbta)], [Co2(μ-OH)2(bbta)], [Co2(μ-OH)2(btdd)] (H2btdd = bis(1H-1,2,3-triazolo-[4,5-b],[4′,5′-i])dibenzo [1][4]dioxin), and [Co2(dobdc)] showed high photocatalytic efficiency in CO2 reduction. Co-MOFs with the μ-OH− ligands adjacent to the open Co centres exhibited high selectivity to CO in the reaction under visible light (420 nm). The ligands acted as hydrogen bond donors and stabilized the primary Co–CO2 adduct. They also provided protons to enable C–O bond breaking. TOF of [Co2(μ-OH)2(btdd)] had the highest value of 0.059 s−1, while maintaining high selectivity toward CO up to 98.2% [76].
5. Photocatalytic Oxidation of Organic Compounds
Recently, huge amounts of dangerous organic contaminants e.g., biphenyls or phenols that are generated in diverse industries enter water reservoirs threatening wildlife and people. Due to the fact that these compounds are non-biodegradable and stable, photocatalytic oxidation processes as an effective method for eliminating resistant pollutants are applied. Besides low energy consumption, mild conditions of the reaction, and broad scope of applications, with this strategy organic contaminants can be decomposed into biodegradable and less toxic molecules or mineralized into carbon dioxide and water. Furthermore, photocatalytic oxidation may be utilized to obtain intermediates that are subsequently used for synthesizing valuable compounds e.g., drugs [77]. Conducting the reactions under light irradiation allows to avoid using high temperatures and consequently unwanted by-products [78]. Cobalt-based MOFs display great photocatalytic performance in oxidation reactions, which results from the activity of cobalt ions, thus they are readily used [79]. The low selectivity is the main limiting factor that has an influence on reaction efficiency. Despite putting great efforts into proving the feasibility, photocatalytic oxidation of organic compounds is often a non-selective process. For this reason, it is crucial to apply photocatalysts, such as Co-MOFs, to obtain products with high selectivity [80].
Hollow structural Co-MOF-74 (h-Co-MOF-74) possessing a thin shell (~50 nm) assembled by ultra-small nanoparticles (8–18 nm) was synthesized and applied in thioanisole oxidation to obtain methyl phenyl sulfoxide (a crucial intermediate for the pharmaceuticals syntheses) [81]. This unique structure carries numerous benefits including high specific surface area, abundant oxygen vacancies, improved ability of light absorption, and easily accessible catalytic active sites. Owing to these properties, the full conversion of thioanisole with 99% selectivity toward methyl phenyl sulfoxide was achieved in ten hours under simulated sunlight irradiation. Almost identical results (99% conversion and 98% selectivity) were obtained after the fifth reuse cycle.
Cobalt(II) was introduced into the metal–organic framework TMU-22(Zn) through post-synthetic exchange, forming the mixed-metal TMU-22(Zn/Co) subsequently applied in benzyl alcohol oxidation to benzaldehyde [82]. Three hours of visible light irradiation yielded 65% efficiency, while with the TMU-22(Zn) only ~25% conversion was reported. Co2+ ions were profitably substituted in the framework at more available positions toward the substrate, hence the catalytic performance was improved. According to the reaction mechanism, the exchange between Co2+ and Zn2+ resulted in the development of a new level in the conduction band. Thereby, the electrons from the valence band may be promoted to this level and, as a consequence, the band gap is narrowed. Cobalt also acts as a trap immobilizing electron, which is then transferred to O2 generating highly oxidative •O2−. Subjected to five oxidation cycles, TMU-22(Zn/Co) showed 54% effectiveness which indicates the possibility of its reuse.
In summary, the application of Co-based MOFs as photocatalysts in the oxidation of numerous alcohols and other organic compounds to corresponding products has been described. Under light irradiation, these materials enable high conversion efficiencies, in addition to selectivity, hence they are gaining more and more attention (Table 2). With the use of Co-MOFs, the oxidation processes can be carried out in mild conditions. The products of this reaction may be applied in the synthesis of e.g., drugs and cosmetics. Furthermore, by decomposition of diverse organic pollutants, less harmful and more easily biodegradable compounds are produced, therefore MOFs may be utilized in removing resistant contaminants from water. Considering the availability, high activity, satisfactory stability, and efficiency of cobalt MOFs as well as their reusability without a significant decrease in the catalytic performance, these materials display excellent potential in the oxidation of organic compounds.
| Photocatalyst | Compound | Conversion Efficiency [%] |
Time | Ref. |
|---|---|---|---|---|
| Fe3O4@Ni–Co-BDC NPs | Benzyl alcohol | 87 | 3 h | [78] |
| 4-methylbenzyl alcohol | 93 | 3 h | ||
| 4-methoxybenzyl alcohol | 98 | 3 h | ||
| 3-methoxybenzyl alcohol | 90 | 3 h | ||
| h-Co-MOF-74 | Thioanisole | 100 | 10 h | [81] |
| 4-bromothioanisole | 100 | 10 h | ||
| 4-fluorobenzaldehyde | 100 | 10 h | ||
| Methyl p-tolyl sulphide | 100 | 10 h | ||
| 4-chlorothioanisole | 100 | 10 h | ||
| TMU-22(Zn/Co) | Benzyl alcohol | 65 | 3 h | [82] |
| CoFe2O4/Ce-UiO-66 | n-hexanol | ~80 | 4 h | [83] |
| 3-nitrobenzyl alcohol | 90 | 6 h | ||
| 4-nitrobenzyl alcohol | 90 | 6 h | ||
| 4-bromobenzyl alcohol | 82 | 6 h | ||
| TMU-49/CNNSs | Benzyl alcohol | 81 | 4 h | [84] |
| 4-nitrobenzyl alcohol | 83 | 3 h | ||
| 4-bromobenzyl alcohol | 91 | 3 h | ||
| 4-methylbenzyl alcohol | 76 | 3 h | ||
| 4-methoxybenzyl alcohol | 45 | 3 h |
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869.
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378.
- De Richter, R.; Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96.
- Manan, Z.A.; Mohd Nawi, W.N.R.; Wan Alwi, S.R.; Klemeš, J.J. Advances in Process Integration research for CO2 emission reduction—A review. J. Clean. Prod. 2017, 167, 1–13.
- Vasseghian, Y.; Khataee, A.; Dragoi, E.N.; Moradi, M.; Nabavifard, S.; Oliveri Conti, G.; Mousavi Khaneghah, A. Pollutants degradation and power generation by photocatalytic fuel cells: A comprehensive review. Arab. J. Chem. 2020, 13, 8458–8480.
- Saeed, M.; Muneer, M.; ul Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2021, 29, 293–311.
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985.
- Zhao, W.; Chen, Z.; Yang, X.; Qian, X.; Liu, C.; Zhou, D.; Sun, T.; Zhang, M.; Wei, G.; Dissanayake, P.D.; et al. Recent advances in photocatalytic hydrogen evolution with high-performance catalysts without precious metals. Renew. Sustain. Energy Rev. 2020, 132, 110040.
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546.
- Lin, S.; Huang, H.; Ma, T.; Zhang, Y. Photocatalytic Oxygen Evolution from Water Splitting. Adv. Sci. 2021, 8, 2002458.
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935.
- Xu, S.; Carter, E.A. Theoretical Insights into Heterogeneous (Photo)electrochemical CO2 Reduction. Chem. Rev. 2019, 119, 6631–6669.
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619.
- Wuttke, S. Introduction to Reticular Chemistry. Metal–Organic Frameworks and Covalent Organic Frameworks by Omar M. Yaghi, Markus J. Kalmutzki, and Christian S. Diercks.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Volume 58, ISBN 3527345027.
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.K.J.; Wuttke, S. Controlling the morphology of metal-organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422.
- Ejsmont, A.; Andreo, J.; Lanza, A.; Galarda, A.; Macreadie, L.; Wuttke, S.; Canossa, S.; Ploetz, E.; Goscianska, J. Applications of reticular diversity in metal–organic frameworks: An ever-evolving state of the art. Coord. Chem. Rev. 2021, 430, 213655.
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
- Hao, Y.-C.; Chen, L.-W.; Li, J.; Guo, Y.; Su, X.; Shu, M.; Zhang, Q.; Gao, W.-Y.; Li, S.; Yu, Z.-L.; et al. Metal-organic framework membranes with single-atomic centers for photocatalytic CO2 and O2 reduction. Nat. Commun. 2021, 12, 2682.
- Su, Y.; Song, Z.; Zhu, W.; Mu, Q.; Yuan, X.; Lian, Y.; Cheng, H.; Deng, Z.; Chen, M.; Yin, W.; et al. Visible-Light Photocatalytic CO2 Reduction Using Metal-Organic Framework Derived Ni(OH)2 Nanocages: A Synergy from Multiple Light Reflection, Static Charge Transfer, and Oxygen Vacancies. ACS Catal. 2021, 11, 345–354.
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal-Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057.
- Ma, S.; Zhou, H.C. A metal-organic framework with entatic metal centers exhibiting high gas adsorption affinity. J. Am. Chem. Soc. 2006, 128, 11734–11735.
- Au, V.K.M. Recent Advances in the Use of Metal-Organic Frameworks for Dye Adsorption. Front. Chem. 2020, 8, 708.
- Mandade, P. Introduction, basic principles, mechanism, and challenges of photocatalysis. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 137–154.
- Li, X.; Yu, J.; Gosztola, D.J.; Fry, H.C.; Deria, P. Wavelength-Dependent Energy and Charge Transfer in MOF: A Step toward Artificial Porous Light-Harvesting System. J. Am. Chem. Soc. 2019, 141, 16849–16857.
- Xiao, T.; Zhong, W.; Zhou, L.; Xu, L.; Sun, X.Q.; Elmes, R.B.P.; Hu, X.Y.; Wang, L. Artificial light-harvesting systems fabricated by supramolecular host–guest interactions. Chin. Chem. Lett. 2019, 30, 31–36.
- Zhao, X.; Song, X.; Li, Y.; Chang, Z.; Chen, L. Targeted Construction of Light-Harvesting Metal-Organic Frameworks Featuring Efficient Host-Guest Energy Transfer. ACS Appl. Mater. Interfaces 2018, 10, 5633–5640.
- Whelan, É.; Steuber, F.W.; Gunnlaugsson, T.; Schmitt, W. Tuning photoactive metal–organic frameworks for luminescence and photocatalytic applications. Coord. Chem. Rev. 2021, 437, 213757.
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2018, 52, 356–366.
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks toward Applications. Adv. Funct. Mater. 2021, 31, 2006291.
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512.
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972.
- Reddy, C.V.; Reddy, K.R.; Harish, V.V.N.; Shim, J.; Shankar, M.V.; Shetti, N.P.; Aminabhavi, T.M. Metal-organic frameworks (MOFs)-based efficient heterogeneous photocatalysts: Synthesis, properties and its applications in photocatalytic hydrogen generation, CO2 reduction and photodegradation of organic dyes. Int. J. Hydrogen Energy 2020, 45, 7656–7679.
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128.
- Tran, N.T.; Trung, L.G.; Nguyen, M.K. The degradation of organic dye contaminants in wastewater and solution from highly visible light responsive ZIF-67 monodisperse photocatalyst. J. Solid State Chem. 2021, 300, 122287.
- Yuan, C.; Cheng, P.; Li, J.; Gao, X.; Gao, X.; Wang, X.; Jin, M.; Nötzel, R.; Zhou, G.; Zhang, Z.; et al. ZIF-67 with Argon annealing treatment for visible light responsive degradation of organic dyes in a wide pH range. Microporous Mesoporous Mater. 2019, 285, 13–20.
- Guo, X.; Liu, L.; Xiao, Y.; Mehmood, R.; Xiao, Y.; Qi, Y.; Zhang, F. Water-Stable Cobalt-Based MOF for Water Oxidation in Neutral Aqueous Solution: A Case of Mimicking the Photosystem II. Inorg. Chem. 2021, 60, 1790–1796.
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257.
- Huang, G.-F.; Ma, Z.-L.; Huang, W.-Q.; Tian, Y.; Jiao, C.; Yang, Z.-M.; Wan, Z.; Pan, A. Semiconductor Photocatalyst: Possibilities and Challenges. J. Nanomater. 2013, 2013, 371356.
- Yemmireddy, V.K.; Hung, Y.-C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety-Opportunities and Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631.
- Sohrabi, S.; Keshavarz Moraveji, M.; Iranshahi, D. A review on the design and development of photocatalyst synthesis and application in microfluidic reactors: Challenges and opportunities. Rev. Chem. Eng. 2020, 36, 687–722.
- Kallawar, G.A.; Barai, D.P.; Bhanvase, B.A. Bismuth titanate based photocatalysts for degradation of persistent organic compounds in wastewater: A comprehensive review on synthesis methods, performance as photocatalyst and challenges. J. Clean. Prod. 2021, 318, 128563.
- Shi, Y.; Yang, A.-F.; Cao, C.-S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production from water. Coord. Chem. Rev. 2019, 390, 50–75.
- Wang, C.; Xie, Z.; Dekrafft, K.E.; Lin, W. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454.
- Lionet, Z.; Kim, T.-H.; Horiuchi, Y.; Lee, S.W.; Matsuoka, M. Linker Engineering of Iron-Based MOFs for Efficient Visible-Light-Driven Water Oxidation Reaction. J. Phys. Chem. C 2019, 123, 27501–27508.
- Dong, Y.-J.; Liao, J.-F.; Kong, Z.-C.; Xu, Y.-F.; Chen, Z.-J.; Chen, H.-Y.; Kuang, D.-B.; Fenske, D.; Su, C.-Y. Conformal coating of ultrathin metal-organic framework on semiconductor electrode for boosted photoelectrochemical water oxidation. Appl. Catal. B Environ. 2018, 237, 9–17.
- Younus, H.A.; Ahmad, N.; Chughtai, A.H.; Vandichel, M.; Busch, M.; Van Hecke, K.; Yusubov, M.; Song, S.; Verpoort, F. A Robust Molecular Catalyst Generated In Situ for Photo- and Electrochemical Water Oxidation. ChemSusChem 2017, 10, 862–875.
- Hong, D.; Jung, J.; Park, J.; Yamada, Y.; Suenobu, T.; Lee, Y.M.; Nam, W.; Fukuzumi, S. Water-soluble mononuclear cobalt complexes with organic ligands acting as precatalysts for efficient photocatalytic water oxidation. Energy Environ. Sci. 2012, 5, 7606–7616.
- Liu, S.; Lei, Y.J.; Xin, Z.J.; Xiang, R.J.; Styring, S.; Thapper, A.; Wang, H.Y. Ligand modification to stabilize the cobalt complexes for water oxidation. Int. J. Hydrogen Energy 2017, 42, 29716–29724.
- Luo, H.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Huang, D.; Lai, C.; Cheng, M.; Wang, W.; Xiong, W.; et al. Recent progress on metal-organic frameworks based- and derived-photocatalysts for water splitting. Chem. Eng. J. 2020, 383, 123196.
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577.
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909.
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414.
- Huang, N.Y.; Shen, J.Q.; Ye, Z.M.; Zhang, W.X.; Liao, P.Q.; Chen, X.M. An exceptionally stable octacobalt-cluster-based metal-organic framework for enhanced water oxidation catalysis. Chem. Sci. 2019, 10, 9859–9864.
- Shah, W.A.; Waseem, A.; Nadeem, M.A.; Kögerler, P. Leaching-free encapsulation of cobalt-polyoxotungstates in MIL-100 (Fe) for highly reproducible photocatalytic water oxidation. Appl. Catal. A Gen. 2018, 567, 132–138.
- Han, J.; Wang, D.; Du, Y.; Xi, S.; Hong, J.; Yin, S.; Chen, Z.; Zhou, T.; Xu, R. Metal-organic framework immobilized cobalt oxide nanoparticles for efficient photocatalytic water oxidation. J. Mater. Chem. A 2015, 3, 20607–20613.
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Lassalle-Kaiser, B.; Mialane, P.; Fontecave, M.; Mellot-Draznieks, C.; Dolbecq, A. A Fully Noble Metal-Free Photosystem Based on Cobalt-Polyoxometalates Immobilized in a Porphyrinic Metal-Organic Framework for Water Oxidation. J. Am. Chem. Soc. 2018, 140, 3613–3618.
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Haouas, M.; Benseghir, Y.; Pino, T.; Ha-Thi, M.H.; Landrot, G.; Mialane, P.; Fontecave, M.; et al. Thin Films of Fully Noble Metal-Free for Photocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 47837–47845.
- Guo, C.; Guo, J.; Zhang, Y.; Wang, D.; Zhang, L.; Guo, Y.; Ma, W.; Wang, J. Synthesis of core-shell catalyst with controllable shell thickness and enhanced photocatalytic activity for visible light-driven water oxidation. CrystEngComm 2018, 20, 7659–7665.
- Dong, Y.; Tian, T.; Xu, C.; Ma, K.; Sun, W.; Ding, Y. Cubic Co-Co prussian blue MOF-based transition metal phosphide as an efficient catalyst for visible light-driven water oxidation. J. Catal. 2020, 382, 13–21.
- Yang, S.; Pattengale, B.; Kovrigin, E.L.; Huang, J. Photoactive Zeolitic Imidazolate Framework as Intrinsic Heterogeneous Catalysts for Light-Driven Hydrogen Generation. ACS Energy Lett. 2017, 2, 75–80.
- Nasalevich, M.A.; Becker, R.; Ramos-Fernandez, E.V.; Castellanos, S.; Veber, S.L.; Fedin, M.V.; Kapteijn, F.; Reek, J.N.H.; van der Vlugt, J.I.; Gascon, J. 2-MIL-125(Ti): Cobaloxime-derived metal–organic framework-based composite for light-driven H2 production. Energy Environ. Sci. 2015, 8, 364–375.
- Yang, G.L.; Che, X.J.; Hou, S.L.; Cao, C.S.; Zhao, B. Photocatalytic Hydrogen Evolution Based on Cobalt-Organic Framework with High Water Vapor Adsorption. Inorg. Chem. 2021, 60, 1922–1929.
- Huo, D.; Lin, F.; Chen, S.; Ni, Y.; Wang, R.; Chen, H.; Duan, L.; Ji, Y.; Zhou, A.; Tong, L. Ruthenium Complex-Incorporated Two-Dimensional Metal–Organic Frameworks for Cocatalyst-Free Photocatalytic Proton Reduction from Water. Inorg. Chem. 2020, 59, 2379–2386.
- Kumar, D.P.; Park, H.; Kim, E.H.; Hong, S.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Noble metal-free metal-organic framework-derived onion slice-type hollow cobalt sulfide nanostructures: Enhanced activity of CdS for improving photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 224, 230–238.
- Avci, G.; Erucar, I.; Keskin, S. Do New MOFs Perform Better for CO2 Capture and H2 Purification? Computational Screening of the Updated MOF Database. ACS Appl. Mater. Interfaces 2020, 12, 41567–41579.
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262.
- Zhang, W.; Huang, R.; Song, L.; Shi, X. Cobalt-based metal-organic frameworks for the photocatalytic reduction of carbon dioxide. Nanoscale 2021, 13, 9075–9090.
- Soni, V.; Xia, C.; Cheng, C.K.; Nguyen, V.-H.; Nguyen, D.L.T.; Bajpai, A.; Kim, S.Y.; Van Le, Q.; Khan, A.A.P.; Singh, P.; et al. Advances and recent trends in cobalt-based cocatalysts for solar-to-fuel conversion. Appl. Mater. Today 2021, 24, 101074.
- Scatena, R.; Guntern, Y.T.; Macchi, P. Electron Density and Dielectric Properties of Highly Porous MOFs: Binding and Mobility of Guest Molecules in Cu3(BTC)2 and Zn3(BTC)2. J. Am. Chem. Soc. 2019, 141, 9382–9390.
- Hoch, L.B.; Szymanski, P.; Ghuman, K.K.; He, L.; Liao, K.; Qiao, Q.; Reyes, L.M.; Zhu, Y.; El-Sayed, M.A.; Singh, C.V. Carrier dynamics and the role of surface defects: Designing a photocatalyst for gas-phase CO2 reduction. Proc. Natl. Acad. Sci. USA 2016, 113, E8011–E8020.
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243.
- Wang, J.W.; Huang, H.H.; Sun, J.K.; Ouyang, T.; Zhong, D.C.; Lu, T.B. Electrocatalytic and Photocatalytic Reduction of CO2 to CO by Cobalt(II) Tripodal Complexes: Low Overpotentials, High Efficiency and Selectivity. ChemSusChem 2018, 11, 1025–1031.
- Wang, F.; Cao, B.; To, W.P.; Tse, C.W.; Li, K.; Chang, X.Y.; Zang, C.; Chan, S.L.F.; Che, C.M. The effects of chelating N4 ligand coordination on Co(II)-catalysed photochemical conversion of CO2 to CO: Reaction mechanism and DFT calculations. Catal. Sci. Technol. 2016, 6, 7408–7420.
- Cho, H.Y.; Yang, D.A.; Kim, J.; Jeong, S.Y.; Ahn, W.S. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 2012, 185, 35–40.
- Wang, Y.; Huang, N.Y.; Shen, J.Q.; Liao, P.Q.; Chen, X.M.; Zhang, J.P. Hydroxide Ligands Cooperate with Catalytic Centers in Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 38–41.
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967.
- Mohammadinezhad, A.; Akhlaghinia, B. Engineered Superparamagnetic Core–Shell Metal–Organic Frame-Work (Fe3O4@Ni–Co-BTC NPs) with Enhanced Photocatalytic Activity for Selective Aerobic Oxidation of Alcohols under Solar Light Irradiation. Catal. Lett. 2021, 151, 107–123.
- Dhakshinamoorthy, A.; Lanzuela, E.M.; Navalon, S.; Garcia, H. Cobalt-based metal organic frameworks as solids catalysts for oxidation reactions. Catalysts 2021, 11, 95.
- Xiong, L.; Tang, J. Strategies and Challenges on Selectivity of Photocatalytic Oxidation of Organic Substances. Adv. Energy Mater. 2021, 11, 2003216.
- Zhang, F.; Zhang, J.; Zhang, B.; Zheng, L.; Cheng, X.; Wan, Q.; Han, B.; Zhang, J. Improved catalytic performance of Co-MOF-74 by nanostructure construction. Green Chem. 2020, 22, 5995–6000.
- Hosseini, S.M.; Dehghan, H.; Safarifard, V. Enhancement of photocatalytic aerobic oxidation of benzyl alcohol with the incorporation of cobalt in Zn-based MOF via post-synthetic metal exchange. Polyhedron 2022, 212, 115581.
- Khosroshahi, N.; Karimi, M.; Taghvaei, T.; Safarifard, V. Ultrasound-assisted synthesis of CoFe2O4/Ce-UiO-66 nanocomposite for photocatalytic aerobic oxidation of aliphatic alcohols. Mater. Today Chem. 2021, 22, 100582.
- Hosseini, S.M.; Karimi, M.; Safarifard, V. Metal-organic framework/carbon nitride nanosheets composites (TMU-49/CNNSs): Efficient photocatalyst for aerobic oxidation of alcohols under visible light. New J. Chem. 2021, 45, 17674–17682.




