Nowadays, materials with great potential for environmental protection are being sought. Metal–organic frameworks, in particular those with cobalt species as active sites, have drawn considerable interest due to their excellent properties. With the use of Co-based metal–organic frameworks (MOFs) as photocatalysts in reactions (dye degradation, water oxidation and splitting, carbon dioxide reduction, in addition to the oxidation of organic compounds), even over 90% degradation efficiencies of various dyes (e.g., methylene blue) can be achieved.
- Co-MOFs
- cobalt active sites
- water oxidation
- CO2 reduction
- light-driven degradation of dyes
1. Introduction
2. Photocatalytic Degradation of Dyes
Textile, paper, and clothing industries are the main cause of the existence of organic dyes in water, which contributes to significant environmental pollution. Industrial effluents that contain toxic and non-biodegradable colorants are highly dangerous toward living organisms, thus their removal from water reservoirs is necessary. Over the past few years, photodegradation has become popular since it carries numerous advantages. The reaction may be conducted at room temperature and lasts only a few hours. Moreover, contaminants can be mineralized to harmless molecules (e.g., water, carbon dioxide) through the in situ generation of radicals without forming harmful secondary products. Light intensity, pH, or adsorption properties can affect the photodegradation of dyes. With the increase in the irradiation intensity, reactive oxygen species are generated at a higher rate and the photocatalytic performance is improved. Electrostatic interactions between the reactants are determined by the pH value, however, each catalyst may work more efficiently at a different pH. Although the moderate dye adsorption enhances the degradation yield, after exceeding a certain limit it can be unfavourable. Not only do fewer photons reach surface active sites, but also the molecules of a dye can act as sensitizers that absorb electrons, scattering them in unwanted directions.
In the first step of the photocatalytic process, pollutants are transported from the environment to the surface of the photocatalyst where oxidation-reduction reactions take place. Photogenerated electrons in the CB and holes in the VB force these reactions to occur. Afterward, the products are desorbed and returned to the liquid phase. In order to generate electron-hole pairs, the energy of the photons must be equal to or exceed the band gap of the photocatalyst [32]. In the photodegradation of dyes, materials such as oxides (TiO2, ZnO), metal salts, or chalcogenides (e.g., CdS, Sb2S3, MnS), including their composites were described, although recently cobalt-based MOFs have been willingly utilized [33]. The large surface area, a wide selection of metallic centres and organic linkers, as well as the possibility to control morphological properties make MOFs excellent potential photocatalysts [33][34].
ZIF-67, synthesized via hydrothermal reaction from cobalt salts, (e.g., Co(NO3)2·6H2O) and 2-methylimidazole as organic linker, has a large specific surface area and is highly porous, hence it exhibits numerous applications in photocatalysis. The mechanism of the photocatalytic degradation of dyes using ZIF-67 is simple (Figure 1). In order to regain the stable state, the electron is taken from the water molecule causing its oxygenation into •OH active form. Additionally, the electron that is present in the LUMO and the oxygen from ZIF-67 surface form •O2− that is subsequently converted into •OH. Dye molecules may be cleaved by these radicals to successfully conduct the photodegradation [35][36].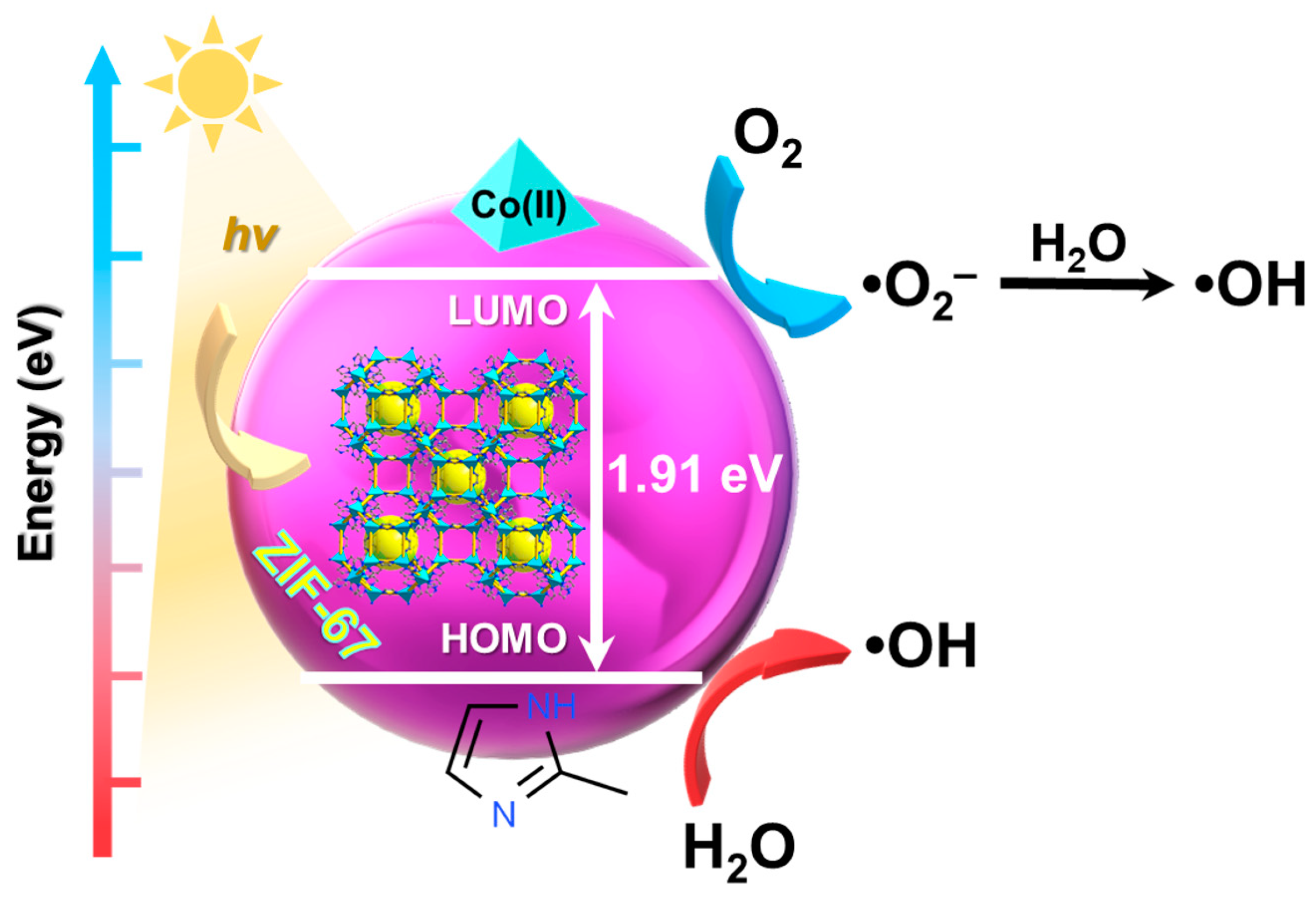
3. Photocatalytic Reactions with Water
3.1. Photocatalytic Water Oxidation
The oxidation of water, also known as the oxygen evolution reaction (OER), is a high energy barrier process that requires the transfer of four electrons. It can be triggered electrochemically or by the use of light. In the photo-driven water oxidation (PWO), the appropriate photosensitizer, catalyst, and sacrificial electron-hole donor are necessary. The purpose of PWO is to artificially mimic photosynthesis, the reactions of which occur on the surface of photocatalytically active materials [37]. The photocatalyst acts as a semiconductor that absorbs light and transfers solar energy to break chemical bonds in H2O. This leads to the generation of the active oxygen species (O•), hydroperoxy species (OOH), and/or metal species (M–O•) at the active metal sites of the catalyst. During this process, oxidation of water with subsequent conversion to O2 takes place, which in acidic media can proceed as follows: 2H2O → O2 + 4e− + 4H+ and in the basic conditions: 4OH− → O2 + 4e− + 2H2O. The oxidation process is also determined by light absorption, charge separation, and catalytic reaction on the surface. The most popular heterogeneous photocatalysts applied in PWO are TiO2, BiVO4, WO3, and α-Fe2O3. However, they encounter challenges related to the impaired four-electron migration kinetics, low light absorption, self-oxidation poisoning by photogenerated holes, and easy recombination of photogenerated charge carriers [38][39][40][41][42]. Hence, there is a great need for novel, highly active, reusable, and stable photocatalysts [10]. MOFs are becoming increasingly popular in photocatalytic water oxidation due to the presence of linkers, capable of absorbing radiation as well as metal nodes that can act as catalytically active sites [43][44][45][46]. Cobalt-based coordination complexes with organic ligands have been intensively studied in photocatalytic water oxidation in the last decade [47]. For instance, [CoII(Me6tren)(OH2)]2+ (Me6tren = tris(N,N′-dimethylaminoethyl)amine), [CoII(12-TMC)]2+ (12-TMC = 1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclododecane), and [CoIII(Cp*)(bpy)(OH2)]2 (Cp* = η5 pentamethylcyclopentadienyl) were tested to evolve O2. However, such complexes were more likely to produce CO2 rather than O2. This was caused by the oxidation of organic ligands and complex decomposition. Hence, cobalt complexes acted more as pre-catalysts, converting into catalytically active Co(OH)x nanoparticles [48]. Although there are reports suggesting ligand modification to stabilize Co-based complexes [49], MOFs have begun to displace them due to their unique properties. The far-reaching symmetry and iterative structure of MOFs, as well as their large specific surface area and increasing stability, have led to greater interest in their photocatalytic application [50].3.2. Photocatalytic Water Splitting
Water splitting is a process of evolving hydrogen and oxygen (2H2O → 2H2 + O2), the main purpose of which is to achieve scalable and cost-viable solar hydrogen production (Figure 2). In recent years, interest in this process has been growing has it does not generate greenhouse gases. One of the main approaches for water splitting and the generation of H2 is to use photocatalysts with appropriate thermodynamic potential, a narrow band gap to harvest light, and an indication of resistance to photocorrosion [51]. Therefore, to enhance the efficiency of photoelectrocatalytic (PEC) water splitting, co-catalysts on semiconductors are often used. They not only provide additional catalytically active sites, but also facilitate redox reactions by suppressing charge recombination and unwanted reverse reactions [52]. At the moment, the quantum efficiency of current photocatalysts is usually less than 10%. There are reports of external quantum efficiencies up to ca. 96% [53], but these are rarely reproduced. Effective photocatalytic water splitting requires complex semiconductor systems coupled with catalysts operating in a broad light spectrum.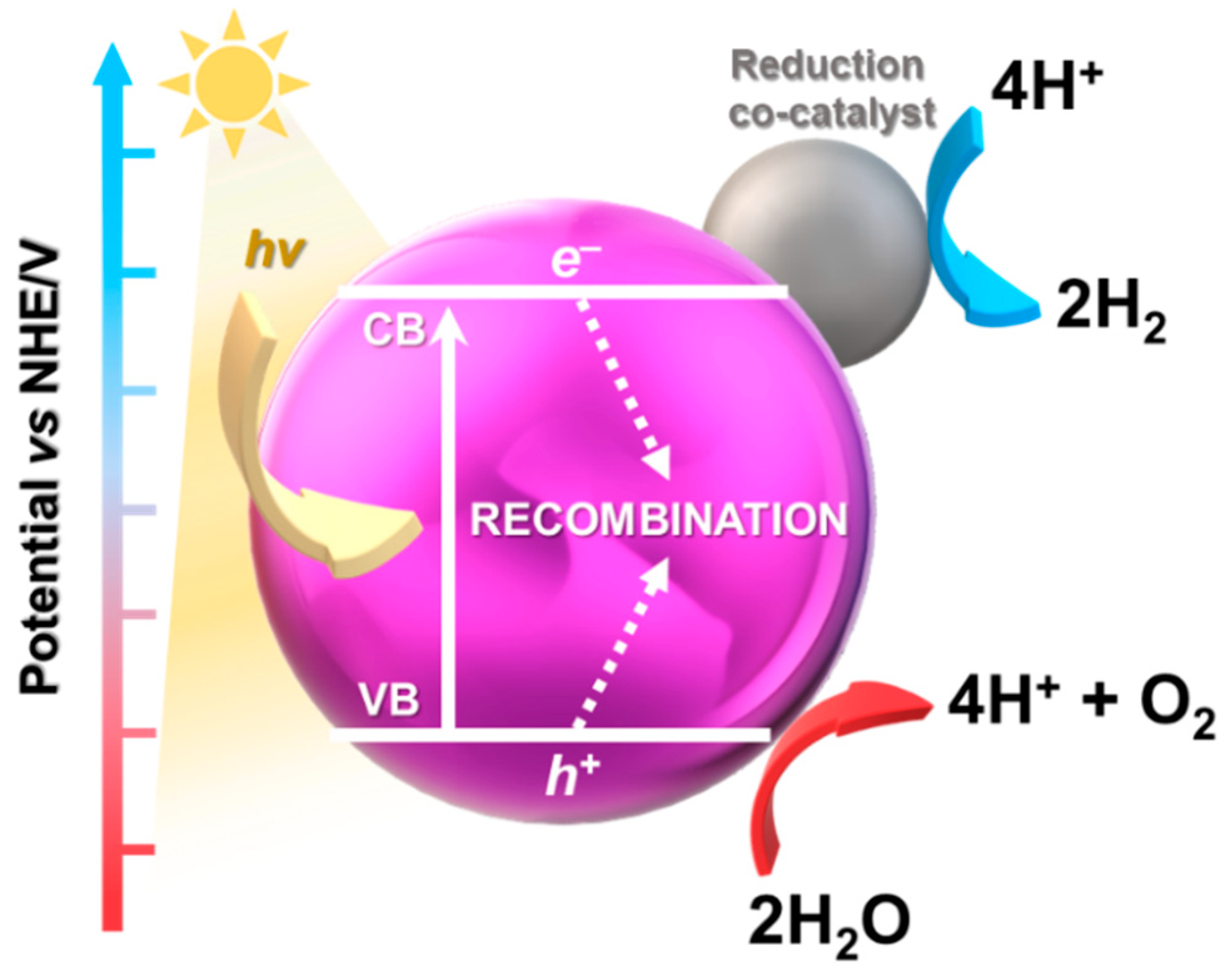
| Photocatalyst | TOF; TON; Generated Product Amount |
Ref. |
|---|---|---|
| Phodo-driven water oxidation | ||
| ZIF-67 | TOF = 0.035 s−11 | [24] |
| Co4-bdt | TOF = 3.050 s−1 | [54] |
| ([CoIICoIIIW11O39(H2O)]−7@MIL-101 | TOF = 0.48 s−1 | [55] |
| [Co4(PW9O34)2(H2O)2]−10@MIL-101 | TOF = 0.53 s−1 | [55] |
| Cox/MIL-101 | TOF = 0.012 s−1 | [56] |
| P2W18Co4@MOF-545 | TOF = 0.040 s−1 | [57] |
| P2W18Co4@MOF-545 film | TON = 1600 | [58] |
| ZIF-67 | 9.8 µmol of O2 | [59] |
| Co-MOF-74@ZIF-67 | 15.0 µmol of O2 | [59] |
| Co-MOF-74 | 11.8 µmol of O2 | [59] |
| CoP@CoOx | 901.5 mmol g−1 h−1 of O2 | [60] |
| Photoelectrocatalytic (PEC) water splitting for hydrogen generation | ||
| ZIF-67 film | 48.5 μmol g−1 of H2 | [61] |
| Cox/NH2-MIL-125(Ti) | TOF = 0.8 h−1 | [62] |
| Co3-XL | 23.05 µmol g−1 h−1 of H2 | [63] |
| (Co0.5[Ru(tpyCOO)2]PF6) | 589 μmol g−1 h−1 of H2 | [64] |
| Co4S3/CdS | 12 360 µmol g−1 h−1 of H2 | [65] |
4. Photocatalytic Carbon Dioxide Reduction
MOFs address many problems associated with photocatalytic processes due to their unique features. In the case of reaction with gases, MOFs show high potential in CO2 capture [66]. If the high adsorption capacity is properly coupled with the photoelectrocatalytic properties of MOFs, it can result in high-performance materials [67]. The general mechanism of CO2 reduction with MOFs proceeds in the following manner. In the beginning, light excites a photosensitizer, which then undergoes reductive quenching by a sacrificial electron donor. The reduced photosensitizer donates its electrons to the MOF with adsorbed CO2. At this stage, the CO2 is reduced to CO2*. Subsequently, the CO2* accepts protons generated from the decomposition of H2O and forms an COOH* intermediate to be further reduced to CO*. Then, CO* is desorbed, resulting in the formation of CO [68].
In the development of materials for CO2 reduction, systems capable of generating multielectrons are required [69]. For a MOF as a catalyst, the LUMO must be above the redox potential for the CO2 reduction half-reaction, which depends on the resulting product. For by far the most common CO2-to-CO conversion via Co-based MOF photocatalysts, the reduction potential is −0.53 V. By tuning the MOF composition, one can influence the optical and electronic responses i.e., HOMO and LUMO energy levels. In addition, the sorption capacity of the MOF is very important. Therefore, its textural parameters (e.g., surface area, pore volume) and improved affinity towards CO2 (e.g., by amine functionalities) must be attained in order to proceed efficient photoreduction [70]. For an effective CO2 conversion process, accessibility to the active sites is vital, thus 2D nanosheet MOFs with better exposed active sites are preferred. Defect engineering also contributes to improved access to nodes. Furthermore, defects play an important role in the excited state and charge relaxation pathways. Higher defect concentrations lead to longer excited-state lifetimes. This is attributed to the trapping of electrons in oxygen vacancies and holes in hydroxyl groups at the surface, which have been shown to play an important role in the CO2 reduction mechanism [71]. The conversion of CO2 can often lead to the formation of many products, but also to the generation of hydrogen from water. Therefore, it is quite challenging to design a material that has high selectivity for a single product without carrying out trial studies. The selectivity of Co-MOFs to receive one type of product in excess is still unclear. It requires a combination of theoretical calculations and the compilation of many parameters that represent a large system of variables during the process. In general, for any cobalt-based semiconductor, it is important to study the light intensity and photon energy, which must be applied to excite semiconductors. The photo-generated electrons and holes affect the kinetics of the reduction rate and ultimately the product selectivity. By attempting to select appropriate co-catalysts, it is possible to mitigate their effect on selectivity. Additionally, a key step is the desorption of the product from the material, which, if hindered, can result in low yields and poor selectivity. In photocatalytic CO2 conversion, it is necessary to couple a gas chromatography system equipped with a thermal conductivity detector (TCD), a hydrogen flame ionization detector (FID), and mass spectrometry to rigorously monitor the reaction products. By combining excitation features, catalyst and co-catalyst band structures, charge separation efficiency, and reactions on the material surface, it will be attainable to control product selectivity and material efficiency [72].
Cobalt-based complexes e.g., cobalt(II) tripodal or tetradentate ligand complexes, have been considered efficient catalysts of CO2 reduction with promising results in terms of high selectivity (95–100%) [73][74]. Most importantly, MOFs are characterized by high CO2 sorption capacities, which could be a key aspect for the implementation of these materials on a larger scale. One of the highest CO2 adsorption capacities of 288 mg g−1 was reported for Co-MOF-74 and attributed to its well-developed surface area of 1314 m2 g−1 [75]. Therefore, the photocatalytic activities of Co-MOFs were tested in CO2 reduction process. For example, Co-based MOFs [Co2(μ-Cl)2(bbta)], [Co2(μ-OH)2(bbta)], [Co2(μ-OH)2(btdd)] (H2btdd = bis(1H-1,2,3-triazolo-[4,5-b],[4′,5′-i])dibenzo [1][4]dioxin), and [Co2(dobdc)] showed high photocatalytic efficiency in CO2 reduction. Co-MOFs with the μ-OH− ligands adjacent to the open Co centres exhibited high selectivity to CO in the reaction under visible light (420 nm). The ligands acted as hydrogen bond donors and stabilized the primary Co–CO2 adduct. They also provided protons to enable C–O bond breaking. TOF of [Co2(μ-OH)2(btdd)] had the highest value of 0.059 s−1, while maintaining high selectivity toward CO up to 98.2% [76].
5. Photocatalytic Oxidation of Organic Compounds
Recently, huge amounts of dangerous organic contaminants e.g., biphenyls or phenols that are generated in diverse industries enter water reservoirs threatening wildlife and people. Due to the fact that these compounds are non-biodegradable and stable, photocatalytic oxidation processes as an effective method for eliminating resistant pollutants are applied. Besides low energy consumption, mild conditions of the reaction, and broad scope of applications, with this strategy organic contaminants can be decomposed into biodegradable and less toxic molecules or mineralized into carbon dioxide and water. Furthermore, photocatalytic oxidation may be utilized to obtain intermediates that are subsequently used for synthesizing valuable compounds e.g., drugs [77]. Conducting the reactions under light irradiation allows to avoid using high temperatures and consequently unwanted by-products [78]. Cobalt-based MOFs display great photocatalytic performance in oxidation reactions, which results from the activity of cobalt ions, thus they are readily used [79]. The low selectivity is the main limiting factor that has an influence on reaction efficiency. Despite putting great efforts into proving the feasibility, photocatalytic oxidation of organic compounds is often a non-selective process. For this reason, it is crucial to apply photocatalysts, such as Co-MOFs, to obtain products with high selectivity [80].
Hollow structural Co-MOF-74 (h-Co-MOF-74) possessing a thin shell (~50 nm) assembled by ultra-small nanoparticles (8–18 nm) was synthesized and applied in thioanisole oxidation to obtain methyl phenyl sulfoxide (a crucial intermediate for the pharmaceuticals syntheses) [81]. This unique structure carries numerous benefits including high specific surface area, abundant oxygen vacancies, improved ability of light absorption, and easily accessible catalytic active sites. Owing to these properties, the full conversion of thioanisole with 99% selectivity toward methyl phenyl sulfoxide was achieved in ten hours under simulated sunlight irradiation. Almost identical results (99% conversion and 98% selectivity) were obtained after the fifth reuse cycle.
Cobalt(II) was introduced into the metal–organic framework TMU-22(Zn) through post-synthetic exchange, forming the mixed-metal TMU-22(Zn/Co) subsequently applied in benzyl alcohol oxidation to benzaldehyde [82]. Three hours of visible light irradiation yielded 65% efficiency, while with the TMU-22(Zn) only ~25% conversion was reported. Co2+ ions were profitably substituted in the framework at more available positions toward the substrate, hence the catalytic performance was improved. According to the reaction mechanism, the exchange between Co2+ and Zn2+ resulted in the development of a new level in the conduction band. Thereby, the electrons from the valence band may be promoted to this level and, as a consequence, the band gap is narrowed. Cobalt also acts as a trap immobilizing electron, which is then transferred to O2 generating highly oxidative •O2−. Subjected to five oxidation cycles, TMU-22(Zn/Co) showed 54% effectiveness which indicates the possibility of its reuse.
In summary, the application of Co-based MOFs as photocatalysts in the oxidation of numerous alcohols and other organic compounds to corresponding products has been described. Under light irradiation, these materials enable high conversion efficiencies, in addition to selectivity, hence they are gaining more and more attention (Table 2). With the use of Co-MOFs, the oxidation processes can be carried out in mild conditions. The products of this reaction may be applied in the synthesis of e.g., drugs and cosmetics. Furthermore, by decomposition of diverse organic pollutants, less harmful and more easily biodegradable compounds are produced, therefore MOFs may be utilized in removing resistant contaminants from water. Considering the availability, high activity, satisfactory stability, and efficiency of cobalt MOFs as well as their reusability without a significant decrease in the catalytic performance, these materials display excellent potential in the oxidation of organic compounds.
| Photocatalyst | Compound | Conversion Efficiency [%] |
Time | Ref. |
|---|---|---|---|---|
| Fe3O4@Ni–Co-BDC NPs | Benzyl alcohol | 87 | 3 h | [78] |
| 4-methylbenzyl alcohol | 93 | 3 h | ||
| 4-methoxybenzyl alcohol | 98 | 3 h | ||
| 3-methoxybenzyl alcohol | 90 | 3 h | ||
| h-Co-MOF-74 | Thioanisole | 100 | 10 h | [81] |
| 4-bromothioanisole | 100 | 10 h | ||
| 4-fluorobenzaldehyde | 100 | 10 h | ||
| Methyl p-tolyl sulphide | 100 | 10 h | ||
| 4-chlorothioanisole | 100 | 10 h | ||
| TMU-22(Zn/Co) | Benzyl alcohol | 65 | 3 h | [82] |
| CoFe2O4/Ce-UiO-66 | n-hexanol | ~80 | 4 h | [83] |
| 3-nitrobenzyl alcohol | 90 | 6 h | ||
| 4-nitrobenzyl alcohol | 90 | 6 h | ||
| 4-bromobenzyl alcohol | 82 | 6 h | ||
| TMU-49/CNNSs | Benzyl alcohol | 81 | 4 h | [84] |
| 4-nitrobenzyl alcohol | 83 | 3 h | ||
| 4-bromobenzyl alcohol | 91 | 3 h | ||
| 4-methylbenzyl alcohol | 76 | 3 h | ||
| 4-methoxybenzyl alcohol | 45 | 3 h |
