| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lotfi Bessais | -- | 677 | 2022-04-13 10:12:13 | | | |
| 2 | Lindsay Dong | + 775 word(s) | 1452 | 2022-04-14 17:25:26 | | | | |
| 3 | Lindsay Dong | + 1279 word(s) | 2731 | 2022-04-15 03:37:37 | | | | |
| 4 | Lindsay Dong | + 978 word(s) | 3709 | 2022-04-15 04:12:54 | | | | |
| 5 | Lindsay Dong | + 59 word(s) | 3768 | 2022-04-15 04:31:01 | | | | |
| 6 | Lindsay Dong | -41 word(s) | 3727 | 2022-09-30 10:53:10 | | | | |
| 7 | Lindsay Dong | -9 word(s) | 3718 | 2022-09-30 11:09:21 | | | | |
| 8 | Lindsay Dong | -14 word(s) | 3704 | 2022-09-30 11:22:07 | | | | |
| 9 | Lindsay Dong | -3027 word(s) | 677 | 2023-06-15 04:22:09 | | |
Video Upload Options
The Pr2Co7 compound has interesting magnetic properties, such as a high Curie temperature TC and uniaxial magnetocrystalline anisotropy. It crystallizes in a hexagonal structure (2:7 H) of the Ce2Ni7 type and is stable at relatively low temperatures (Ta ≤ 1023 K), or it has a rhombohedral structure (2:7 R) of the Gd2Co7 type and is stable at high temperatures (Ta ≥ 1223 K). Studies of the magnetocaloric properties of the nanocrystalline Pr2Co7 compound have shown the existence of a large reversible magnetic entropy change (ΔSM) with a second-order magnetic transition.
1. Introduction
electrons of transition metals (T), such as cobalt (Co) and iron (Fe) [24,25,26,27,28,29,30,31,32,33]. The R elements thus provide their strong magnetocrystalline anisotropy (Ha) due to the interactions between their orbital moment and the crystal field. The 3d metals provide their strong magnetization and a high Curie temperature (TC) due to the important exchange interactions between the 3d elements [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Permanent magnets are the idea of an ever-increasing number of recent devices. Alloys and intermetallic compounds obtained by combining (R) elements with metals (T) form a crucial class of materials for which applications have been found in permanent magnets [44]. Among the intermetallic systems, the noncrystalline Pr2Co7 compound is currently one of the most promising [34,52,53,54]. The interest in these systems is due to the combination of the complementary characteristics of the 3d-itinerant and 4f-localized magnetism of Co and Pr atoms, respectively [54,55]. In order to strengthen these interactions, it is necessary to partially substitute the Co atoms in the noncrystalline Pr2Co7 compounds with an appropriate element, such as iron (Fe), or through the insertion of a light element, such as carbon (C) or hydrogen (H), which can increase interatomic distances and strengthen the magnetic moment.
3. Structural, Microstructural, and Magnetic Characterizations
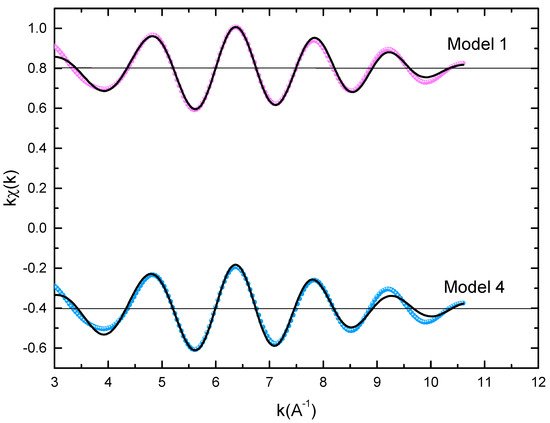
The microstructural characterizations of the Pr2Co7, Pr2Co7−xFex, Pr2CoxCx, and Pr2CoxHx samples were investigated by using X-ray diffraction (XRD; Bruker D8 Advance) with CuKα radiation of wavelength λ = 0.154056 nm. The refinement of the patterns was done by using the FULLPROF computing code based on the Rietveld method [66,67] with the assumption of the Thompson–Cox–Hastings line profile. The goodness-of-fit indicators χ2 and RB
was measured on a MANICS differential sample magnetometer (DSM-871 Magneto/susceptometer) in a field of 1 kOe with a sample of around 5–10 × 10−3 g. TC was obtained from the M(T) curve by extracting the linear part of the M(T) curve and determining the temperature value of the intersection with the expanded baseline. Magnetic hysteresis M(H) loops were determined using a Physical Properties Measurement System (PPMS9) Quantum Design microscope.
4. Structural Properties
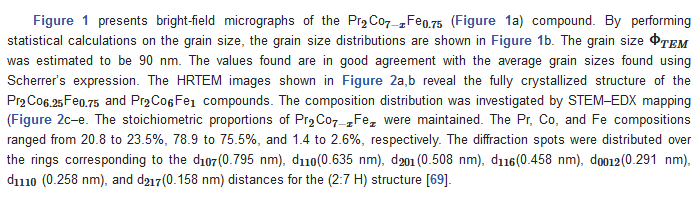
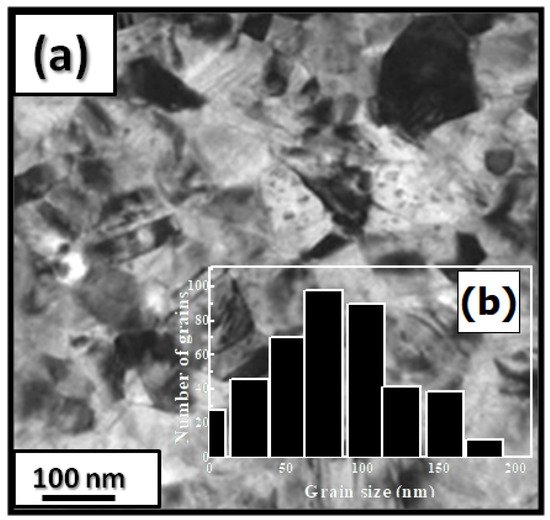
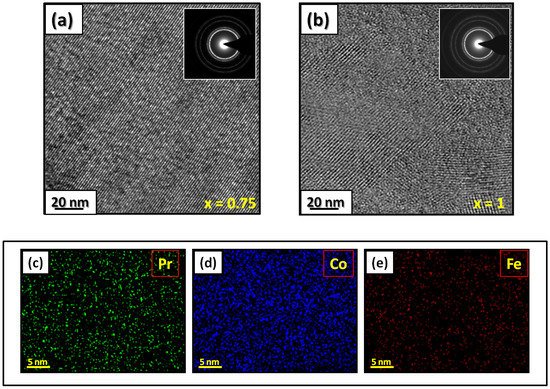
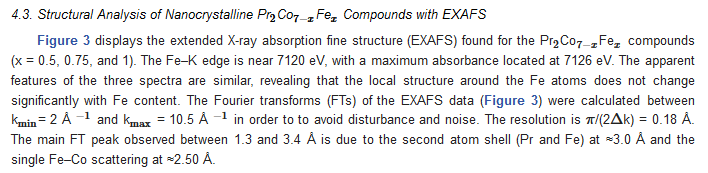
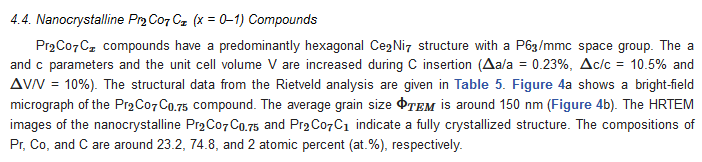
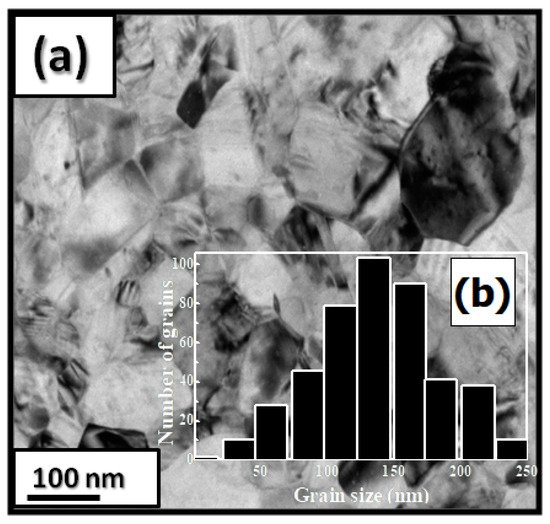
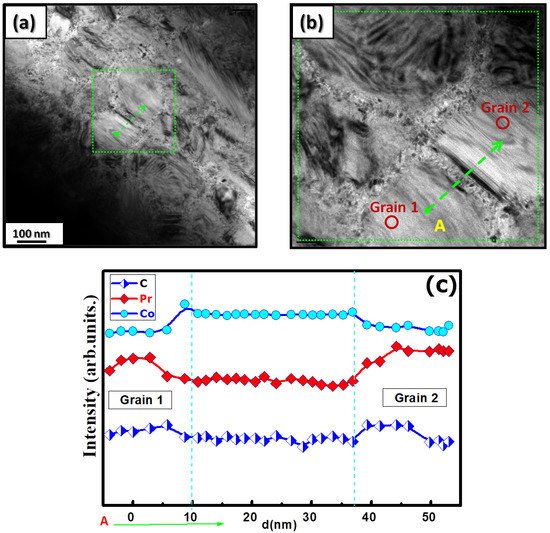
| x | 0.0 | 0.25 | 0.5 | 0.75 | 1 |
|---|---|---|---|---|---|
| a (Å) | 5.068(1) | 5.070(3) | 5.076(1) | 5.079(11) | 5.080(2) |
| c (Å) | 24.456(2) | 24.509(5) | 25.009(3) | 25.576(4) | 26.981(2) |
| c/a | 4.825 | 4.832 | 4.841 | 5.035 | 5.311 |
| V(Å3 |
| ) | 544.02 | 547 | 555 | 567.4 | 598.9 |
| RB |
| 3.1 | 2.43 | 1.31 | 2.76 | 2.42 | |
| χ2 |
| 3.80 | 3.28 | 3.60 | 3.38 | 3.36 |
5. Intrinsic Magnetic Properties
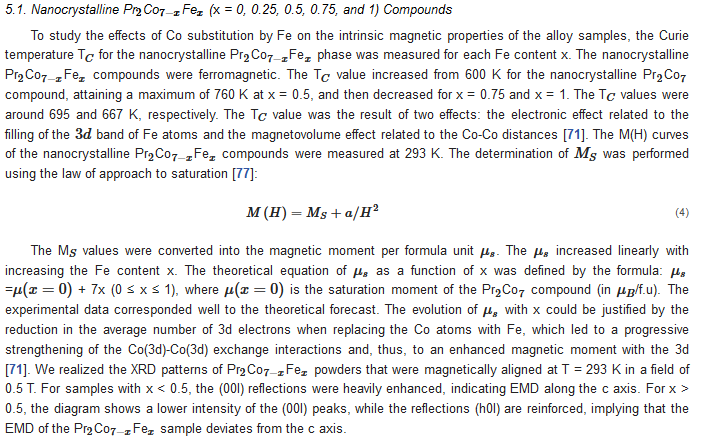
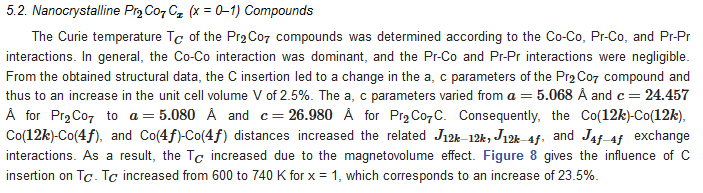
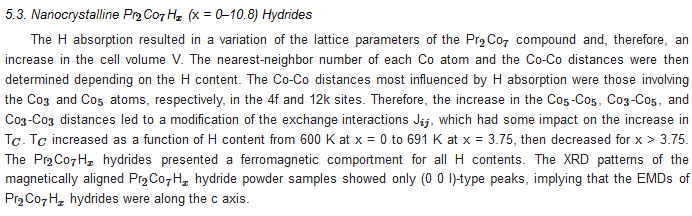
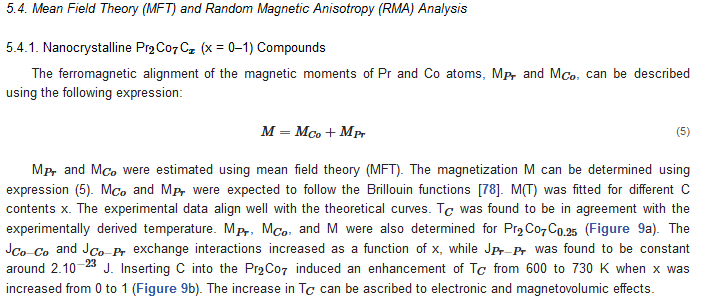

6. Extrinsic Magnetic Properties