The Pr2Co7 compound has interesting magnetic properties, such as a high Curie temperature TC and uniaxial magnetocrystalline anisotropy. It crystallizes in a hexagonal structure (2:7 H) of the Ce2Ni7 type and is stable at relatively low temperatures (Ta ≤ 1023 K), or it has a rhombohedral structure (2:7 R) of the Gd2Co7 type and is stable at high temperatures (Ta ≥ 1223 K). Studies of the magnetocaloric properties of the nanocrystalline Pr2Co7 compound have shown the existence of a large reversible magnetic entropy change (ΔSM) with a second-order magnetic transition.
- magnetic properties
- magnetocaloric properties
- Pr2Co7 Compound
1. Introduction
In recent years, magnetic nanomaterials based on rare-earth elements (R) and transition metals (T) have been widely investigated due to their extremely diverse potential applications in industrial fields [
,
,
,
,
,
,
,
]. These properties are often used to produce soft, hard, or semi-hard magnetic materials [
,
,
,
,13,
,
,
,
,
,
,
,
,
,
]. The origin of these exceptional magnetic properties is particularly due to the coexistence of of two complementary kinds of magnetism: the localized magnetism characteristic of rare-earth (R) electrons and the itinerant magnetism of the electrons of transition metals (T), such as cobalt (Co) and iron (Fe) [24,25,26,27,28,29,30,31,32,33]. The R elements thus provide their strong magnetocrystalline anisotropy (Ha ) due to the interactions between their orbital moment and the crystal field. The 3d metals provide their strong magnetization and a high Curie temperature (TC ) due to the important exchange interactions between the 3d elements [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Permanent magnets are the idea of an ever-increasing number of recent devices. Alloys and intermetallic compounds obtained by combining (R) elements with metals (T) form a crucial class of materials for which applications have been found in permanent magnets [44]. Among the intermetallic systems, the noncrystalline Pr2 Co7 compound is currently one of the most promising [34,52,53,54]. The interest in these systems is due to the combination of the complementary characteristics of the 3d -itinerant and 4f -localized magnetism of Co and Pr atoms, respectively [54,55]. In order to strengthen these interactions, it is necessary to partially substitute the Co atoms in the noncrystalline Pr2 Co7 compounds with an appropriate element, such as iron (Fe), or through the insertion of a light element, such as carbon (C) or hydrogen (H), which can increase interatomic distances and strengthen the magnetic moment.
2. Synthesis Methods of Pr Co , Pr Co Fe , Pr Co C ,and Pr Co H Samples
To prepare the nanocrystalline Pr Co and Pr Co Fe (x = 0.25, 0.5, 0.75, and 1) samples, high-energy ball milling was carried out on previously prepared ingots [60]. The pre-alloy obtained by fusion was broken inside a glove box with a mortar and then introduced with five beads into a hermetically sealed jar. A Fritsch P7 planetary mill with a bead/powder ratio of 15:1 was used to get finer particles. The ground powder was then wrapped in tantalum foil (Ta) and then sealed in quartz ampoules under secondary vacuum (10 bar) at different annealing temperatures: T = 700 K and 1350 K. The tantalum foil (Ta) was used to avoid contamination due to contact with silica; these samples were first degassed under secondary vacuum to ensure that no impurities came to pollute the synthesis products. The sample was cooled after leaving the oven by quenching in water. To insert carbon atoms (C) into the Pr Co elemental cell, we used a carbonation technique that involved a solid–solid-type reaction according to the following reaction [61]:
Hydrogen atoms (H) were inserted into the Pr Co compound with a solid–gas reaction between the sample and 99.99% pure dihydrogen (H ) [58]. The method used was that of Sievert [62,63]. The amount of absorbed hydrogen was determined with a volumetric method [64,65].
3. Structural, Microstructural, and Magnetic Characterizations of the Samples
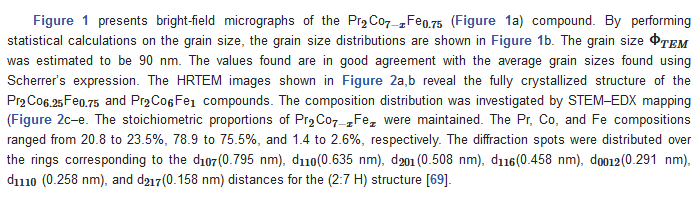
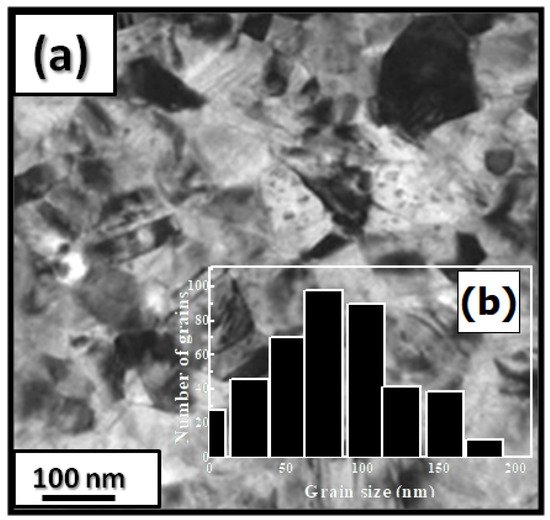
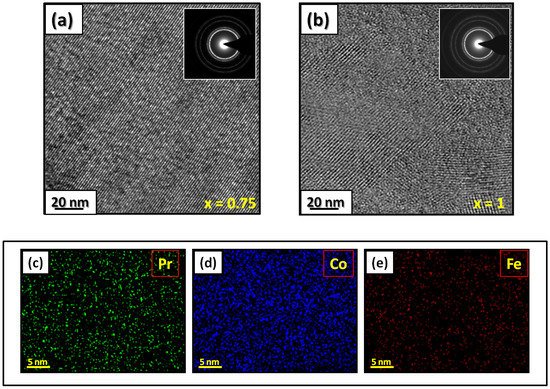
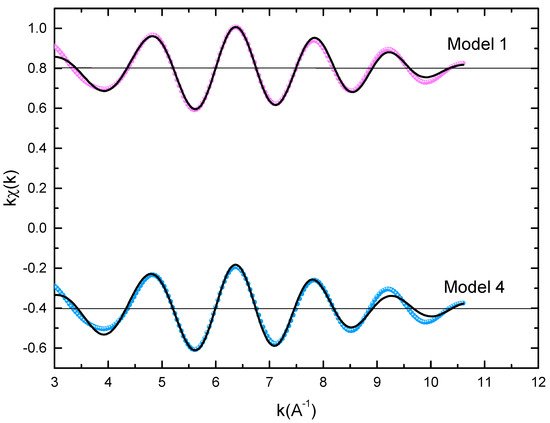
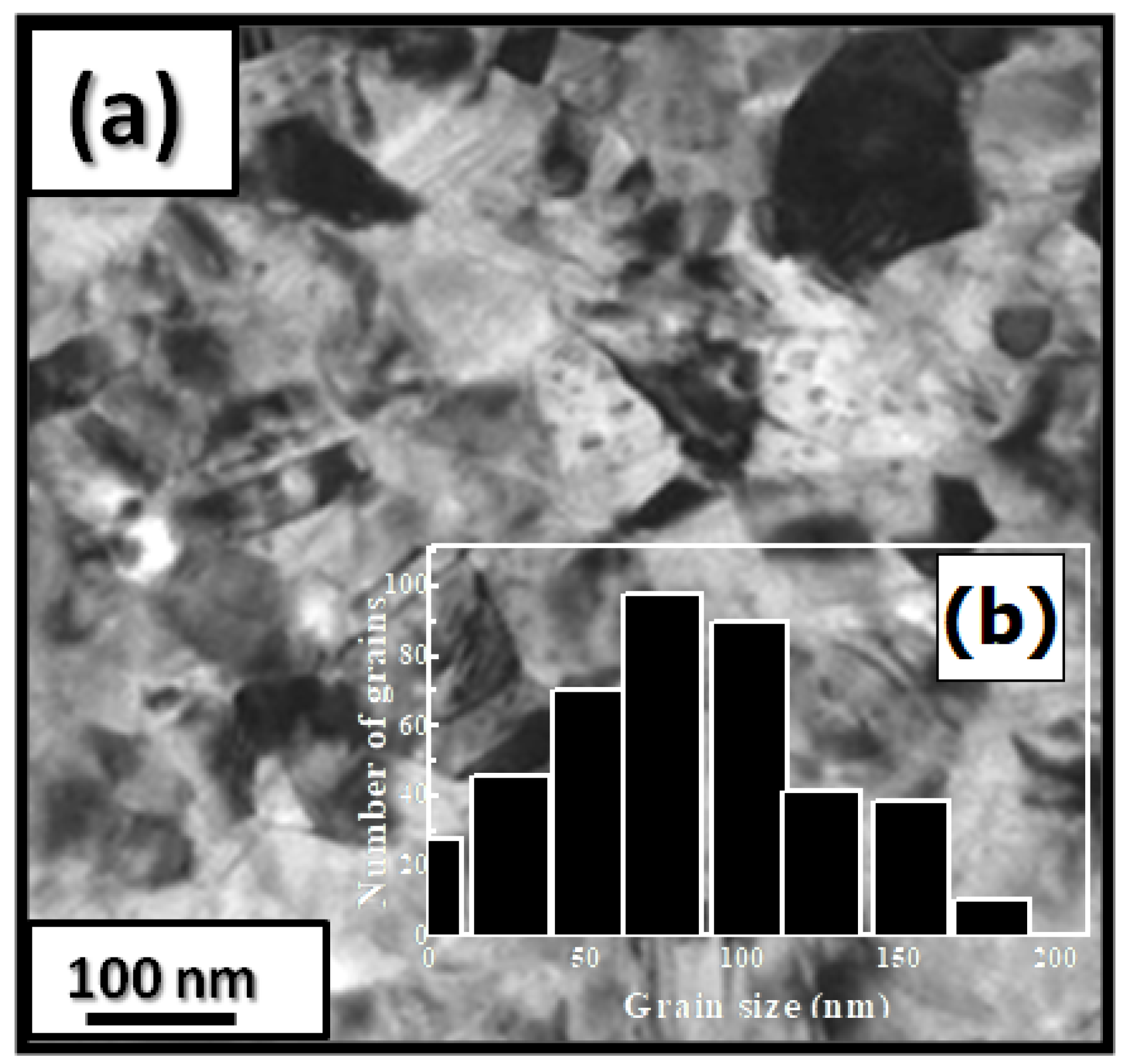
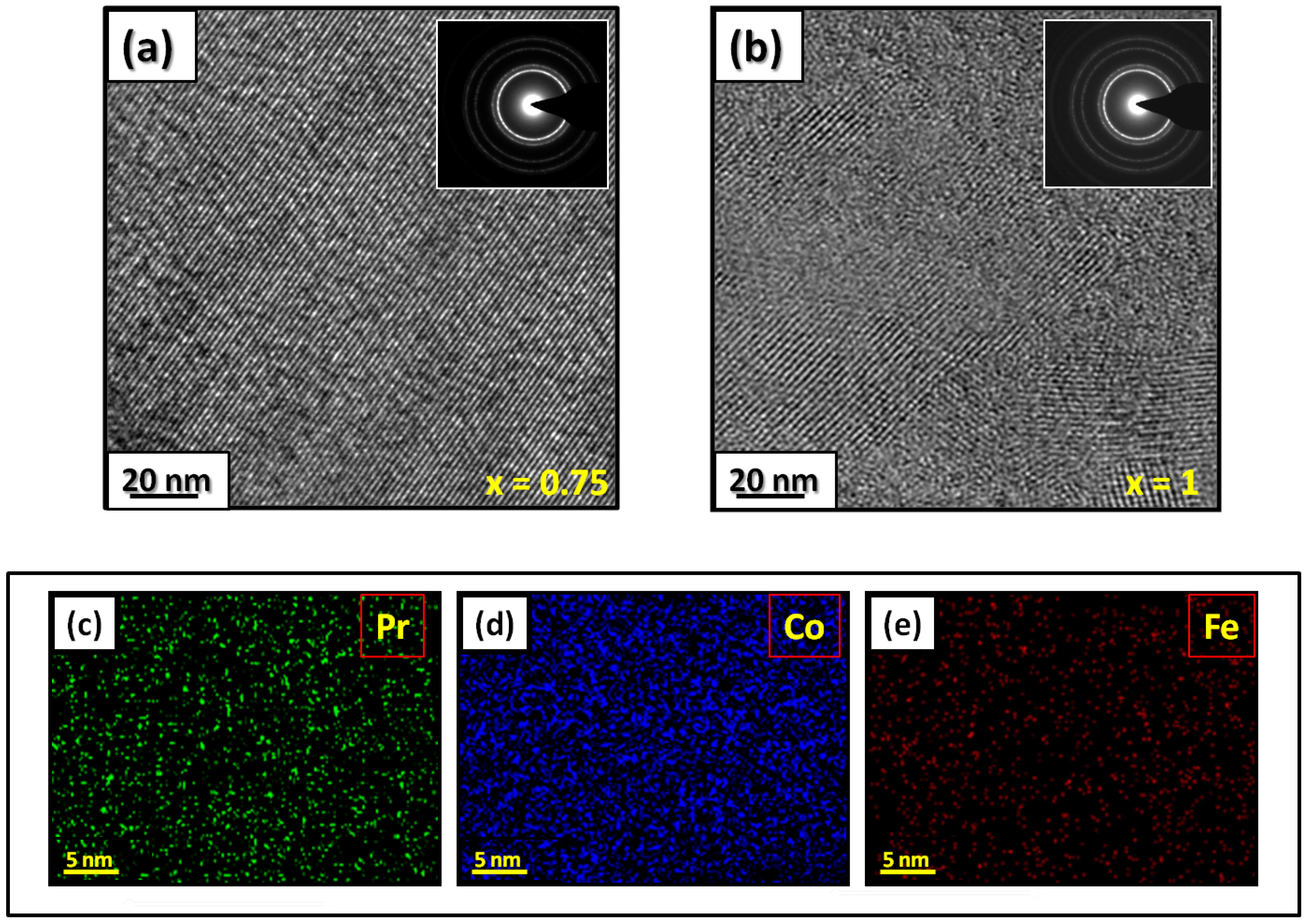
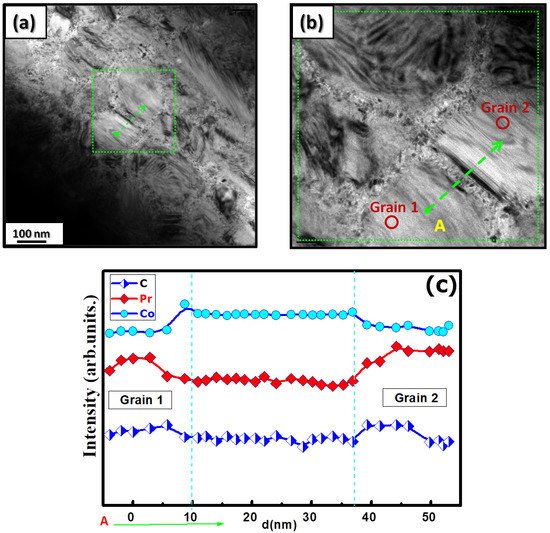
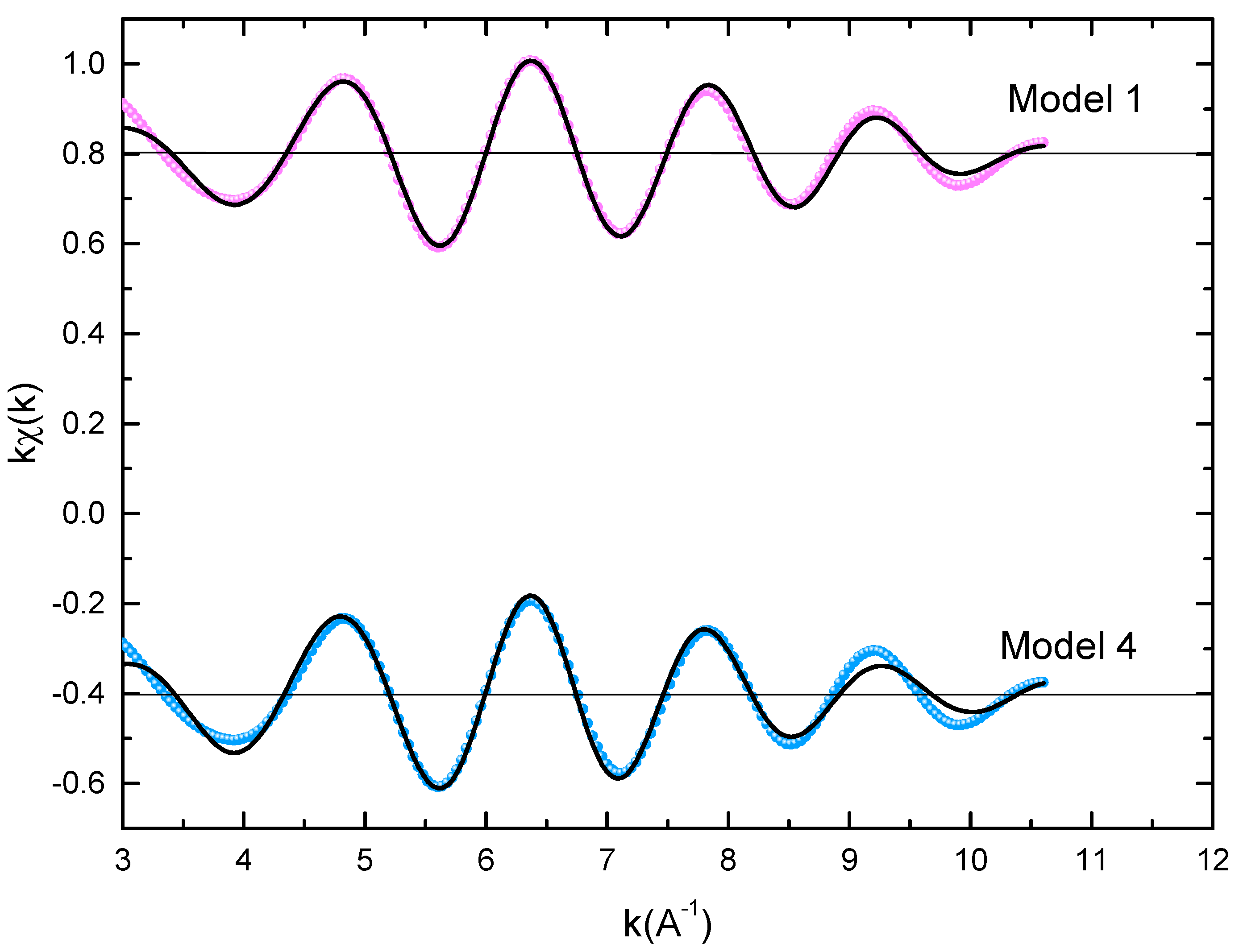
The microstructural characterizations of the Pr2 Co7 , Pr2 Co7−x Fex , Pr2 Cox Cx , and Pr2 Cox Hx samples were investigated by using X-ray diffraction (XRD; Bruker D8 Advance) with CuKα radiation of wavelength λ = 0.154056 nm. The refinement of the patterns was done by using the FULLPROF computing code based on the Rietveld method [66,67] with the assumption of the Thompson–Cox–Hastings line profile. The goodness-of-fit indicators and χ2R were calculated as previously described ind RB [ ]. Extended X-ray absorption fine-structure (EXAFS) measurements were performed on a 2.75 GeV SAMBA beamline, Synchrotron SOLEIL, France. EXAFS experiments were carried out at 293 K in the fluorescence mode using a 4-element Si(111) drift detector. The EXAFS spectra were extrapolated using the MAXeCherokee code [ , ], while the fitting process and comparison between theoretical and experimental EXAFS curves were carried out with the MAX-Roundmidnight package [ ]. The theoretical phases and amplitudes used in the EXAFS models were determined with the FEFF8 code [ ] by using the crystal structure results of the Rietveld refinements at each Fe site with the use of the MAX-CRYSTALFFREV code [ ] for the Crystal Structure–EXAFS Modeling interface. The morphology, the chemical compositions, and the images were considered using a JEOL 2010 FEG transmission electron microscope equipped with an energy-dispersive spectrometer (EDS). For the TEM measurements, specimens were thinned using a focused-ion-beam-type FEI Helios 600 Nanolab dual-beam instrument. The Curie temperature T was measured on a MANICS differential sample magnetometer (DSM-871 Magneto/susceptometer) in a field of 1 kOe with a sample of around 5–10 × 10−3 g. TC was obtained from the M(T) curve by extracting the linear part of the M(T) curve and determining the temperature value of the intersection with the expanded baseline. Magnetic hysteresis M(H) loops were determined using a Physical Properties Measurement System (PPMS9) Quantum Design microscope. The nanocrystalline Pr Co compound can crystallize into two polymorphic structures [54] as a function of the annealing temperature T ; the first is a hexagonal (2:7 H) structure of the Ce Ni type ( space group) that is stable at T ≤ 1023 K with the lattice parameters Å and Å. The second is a rhombohedral (2:7 R) structure of the Gd Co type ( space group) that is stable at Ta ≥ 1223 K. The lattice parameters are Å and c = 36.549 ÅṪhe Pr Co cells can be defined by stacking the hexagonal structural blocks for PrCo (CaCu -type structure) and the cubic PrCo blocks (MgCu - and MgZn -type structures) along the common hexagonal (trigonal for PrCo ) axis [54]. The lattice parameters of the two structures are distinguished by the c parameter, which is higher for the 2:7 R structure due to the difference in stacking ([2H] = [BBA BBA ], [3R ] = 3[BBA ]) [71,72,73]. Figure 1 presents bright-field micrographs of the Pr Co Fe (Figure 1a) compound. By performing statistical calculations on the grain size, the grain size distributions are shown in b. The grain size was estimated to be 90 nm. The values found are in good agreement with the average grain sizes found using Scherrer’s expression. The HRTEM images shown in Figure 2a,b reveal the fully crystallized structure of the Pr Co Fe and Pr Co Fe compounds. The composition distribution was investigated by STEM–EDX mapping ( c–e. The stoichiometric proportions of Pr Co Fe were maintained. The Pr, Co, and Fe compositions ranged from 20.8 to 23.5%, 78.9 to 75.5%, and 1.4 to 2.6%, respectively. The diffraction spots were distributed over the rings corresponding to the d (0.795 nm), d (0.635 nm), d (0.508 nm), d (0.458 nm), d (0.291 nm), d (0.258 nm), and d (0.158 nm) distances for the (2:7 H) structure [69] Bright-field micrography of the Pr Co 6.25 Fe 0.75 compound (a). The grain size distribution is shown in the inset (b). HRTEM images (the selected area of electron diffraction is shown in the inset): ( ) Pr 2 Co 6.25 Fe 0.75 and ( ) Pr Co Fe compounds annealed at 1023 K. Elemental mapping of Pr 2 Co 6.25 Fe 0.75 : (c) Pr, (d) Co and (e) Fe. Figure 3 displays the extended X-ray absorption fine structure (EXAFS) found for the Pr Co Fe compounds (x = 0.5, 0.75, and 1). The Fe–K edge is near 7120 eV, with a maximum absorbance located at 7126 eV. The apparent features of the three spectra are similar, revealing that the local structure around the Fe atoms does not change significantly with Fe content. The Fourier transforms (FTs) of the EXAFS data (Figure 5. 3) were calculated between k 2 Å and k = 10.5 Å − 1 in order to to avoid disturbance and noise. The resolution is /(2 k) = 0.18 Å. The main FT peak observed between 1.3 and 3.4 Å is due to the second atom shell (Pr and Fe) at ≈3.0 Å and the single Fe–Co scattering at ≈2.50 Å. Experimental EXAFS spectra in k space of the imaginary part of the Fourier transforms of the Pr Pr Fe compound, adjusted using Models 1 and 4. Black and red lines show the experimental signal and theoretical curves, respectively.4. Structural Properties
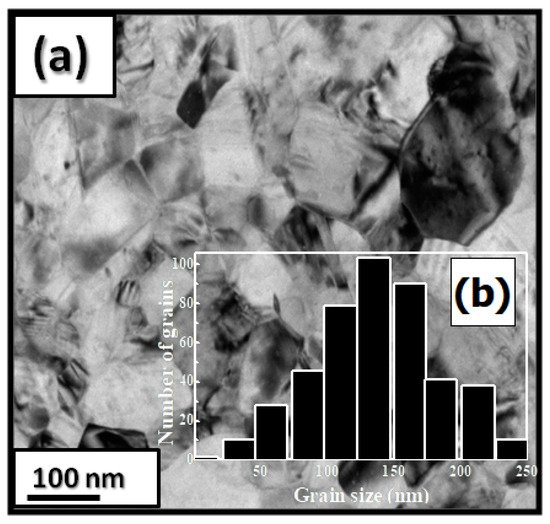
4.1. Nanocrystalline Pr Co Compound
4.2. Nanocrystalline Pr Co Fe (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
4.3. Structural Analysis of Nanocrystalline Pr 2 Co 7 − x Fe x Compounds with EXAFS
x
0.0
0.25
0.5
0.75
1
a (Å)
5.068(1)
5.070(3)
5.076(1)
5.079(11)
5.080(2)
c (Å)
24.456(2)
24.509(5)
25.009(3)
25.576(4)
26.981(2)
c/a
4.825
4.832
4.841
5.035
5.311
V(Å3
)
544.02
547
555
567.4
598.9
RB
3.1
2.43
1.31
2.76
2.42
χ2
3.80
3.28
3.60
3.38
3.36
5. Intrinsic Magnetic Properties
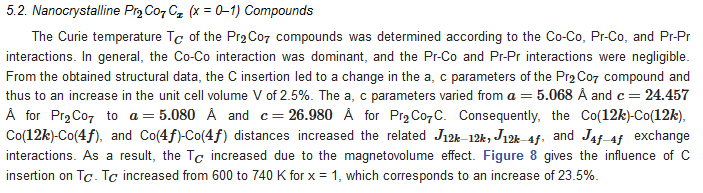
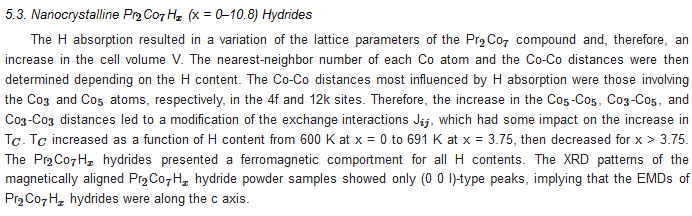
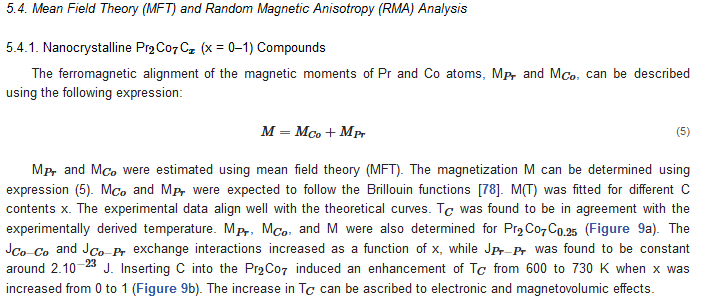
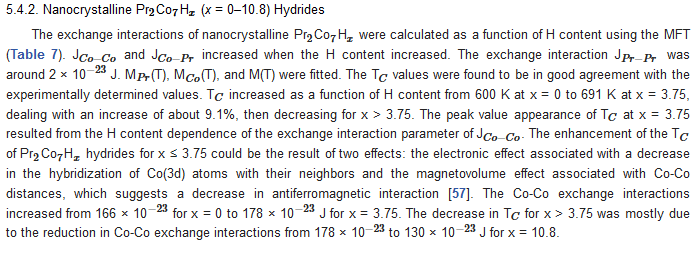

6. Extrinsic Magnetic Properties