The Pr2Co7 compound has interesting magnetic properties, such as a high Curie temperature TC and uniaxial magnetocrystalline anisotropy. It crystallizes in a hexagonal structure (2:7 H) of the Ce2Ni7 type and is stable at relatively low temperatures (Ta ≤ 1023 K), or it has a rhombohedral structure (2:7 R) of the Gd2Co7 type and is stable at high temperatures (Ta ≥ 1223 K). Studies of the magnetocaloric properties of the nanocrystalline Pr2Co7 compound have shown the existence of a large reversible magnetic entropy change (ΔSM) with a second-order magnetic transition.
- magnetic properties
- magnetocaloric properties
- Pr2Co7 Compound
1. Introduction
In recent years, magnetic nanomaterials based on rare-earth elements (R) and transition metals (T) have been widely investigated due to their extremely diverse potential applications in industrial fields [1][2][3][4][5][6][7][8]. These properties are often used to produce soft, hard, or semi-hard magnetic materials [9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]. The origin of these exceptional magnetic properties is particularly due to the coexistence of of two complementary kinds of magnetism: the localized magnetism characteristic of rare-earth (R) electrons and the itinerant magnetism of the electrons of transition metals (T), such as cobalt (Co) and iron (Fe) [24][25][26][27][28][29][30][31][32][33]. The R elements thus provide their strong magnetocrystalline anisotropy (H ) due to the interactions between their orbital moment and the crystal field. The metals provide their strong magnetization and a high Curie temperature (T ) due to the important exchange interactions between the elements [34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51]. Permanent magnets are the idea of an ever-increasing number of recent devices. Alloys and intermetallic compounds obtained by combining (R) elements with metals (T) form a crucial class of materials for which applications have been found in permanent magnets [44]. Among the intermetallic systems, the noncrystalline Pr2Co7 compound is currently one of the most promising [34][52][53][54]. The interest in these systems is due to the combination of the complementary characteristics of the -itinerant and -localized magnetism of Co and Pr atoms, respectively [54][55]. In order to strengthen these interactions, it is necessary to partially substitute the Co atoms in the noncrystalline Pr2Co7 compounds with an appropriate element, such as iron (Fe), or through the insertion of a light element, such as carbon (C) or hydrogen (H), which can increase interatomic distances and strengthen the magnetic moment.
2. Synthesis Methods of Pr2Co7, Pr2Co7−xFex, Pr2CoxCx, and Pr2CoxHx Samples
To prepare the nanocrystalline Pr2Co7 and Pr2Co7−xFex (x = 0.25, 0.5, 0.75, and 1) samples, high-energy ball milling was carried out on previously prepared ingots [56]. The pre-alloy obtained by fusion was broken inside a glove box with a mortar and then introduced with five beads into a hermetically sealed jar. A Fritsch P7 planetary mill with a bead/powder ratio of 15:1 was used to get finer particles. The ground powder was then wrapped in tantalum foil (Ta) and then sealed in quartz ampoules under secondary vacuum (10-6 bar) at different annealing temperatures: T = 700 K and 1350 K. The tantalum foil (Ta) was used to avoid contamination due to contact with silica; these samples were first degassed under secondary vacuum to ensure that no impurities came to pollute the synthesis products. The sample was cooled after leaving the oven by quenching in water. To insert carbon atoms (C) into the Pr2Co7 elemental cell, scholars used a carbonation technique that involved a solid–solid-type reaction according to the following reaction [57]:
Hydrogen atoms (H) were inserted into the Pr2Co7 compound with a solid–gas reaction between the sample and 99.99% pure dihydrogen (H2) [58]. The method used was that of Sievert [59][60]. The amount of absorbed hydrogen was determined with a volumetric method [61][62].
3. Structural, Microstructural, and Magnetic Characterizations of the Samples
The microstructural characterizations of the Pr2Co7, Pr2Co7−xFex, Pr2CoxHX, and Pr2CoxHX samples were investigated by using X-ray diffraction (XRD; Bruker D8 Advance) with CuK radiation of wavelength = 0.154056 nm. The refinement of the patterns was done by using the FULLPROF computing code based on the Rietveld method [63][64] with the assumption of the Thompson–Cox–Hastings line profile. The goodness-of-fit indicators and R were calculated as previously described in [57]. Extended X-ray absorption fine-structure (EXAFS) measurements were performed on a 2.75 GeV SAMBA beamline, Synchrotron SOLEIL, France. EXAFS experiments were carried out at 293 K in the fluorescence mode using a 4-element Si(111) drift detector. The EXAFS spectra were extrapolated using the MAXeCherokee code [65][66], while the fitting process and comparison between theoretical and experimental EXAFS curves were carried out with the MAX-Roundmidnight package [65]. The theoretical phases and amplitudes used in the EXAFS models were determined with the FEFF8 code [67] by using the crystal structure results of the Rietveld refinements at each Fe site with the use of the MAX-CRYSTALFFREV code [65] for the Crystal Structure–EXAFS Modeling interface.
The morphology, the chemical compositions, and the images were considered using a JEOL 2010 FEG transmission electron microscope equipped with an energy-dispersive spectrometer (EDS). For the TEM measurements, specimens were thinned using a focused-ion-beam-type FEI Helios 600 Nanolab dual-beam instrument. The Curie temperature T was measured on a MANICS differential sample magnetometer (DSM-871 Magneto/susceptometer) in a field of 1 kOe with a sample of around 5–10 × 10 g. T was obtained from the M(T) curve by extracting the linear part of the M(T) curve and determining the temperature value of the intersection with the expanded baseline. Magnetic hysteresis M(H) loops were determined using a Physical Properties Measurement System (PPMS9) Quantum Design microscope.
4. Structural Properties
4.1. Nanocrystalline Pr2Co7 Compound
The nanocrystalline Pr2Co7 compound can crystallize into two polymorphic structures [54] as a function of the annealing temperature T ; the first is a hexagonal (2:7 H) structure of the Ce2Ni7 type ( space group) that is stable at T ≤ 1023 K with the lattice parameters Å and Å. The second is a rhombohedral (2:7 R) structure of the Gd2Co7 type ( space group) that is stable at Ta ≥ 1223 K. The lattice parameters are Å and c = 36.549 ÅṪhe Pr2Co7 cells can be defined by stacking the hexagonal structural blocks for PrCo5 (CaCu5-type structure) and the cubic PrCo2 blocks (MgCu2- and MgZn2-type structures) along the common hexagonal (trigonal for PrCo2) axis [54]. The lattice parameters of the two structures are distinguished by the c parameter, which is higher for the 2:7 R structure due to the difference in stacking ([2H] = [BBA BBA ], [3R ] = 3[BBA ]) [68][69][70].
4.2. Nanocrystalline Pr2Co7-xFex (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
Figure 1 presents bright-field micrographs of the Pr2Co7-xFe0.75 (Figure 1a) compound. By performing statistical calculations on the grain size, the grain size distributions are shown in Figure 1b. The grain size was estimated to be 90 nm. The values found are in good agreement with the average grain sizes found using Scherrer’s expression. The HRTEM images shown in Figure 2a,b reveal the fully crystallized structure of the Pr2Co6.25Fe0.75 and Pr2Co6Fe1 compounds. The composition distribution was investigated by STEM–EDX mapping (Figure 2c–e. The stoichiometric proportions of Pr2Co7-xFex were maintained. The Pr, Co, and Fe compositions ranged from 20.8 to 23.5%, 78.9 to 75.5%, and 1.4 to 2.6%, respectively. The diffraction spots were distributed over the rings corresponding to the d (0.795 nm), d (0.635 nm), d (0.508 nm), d (0.458 nm), d (0.291 nm), d (0.258 nm), and d (0.158 nm) distances for the (2:7 H) structure [66]
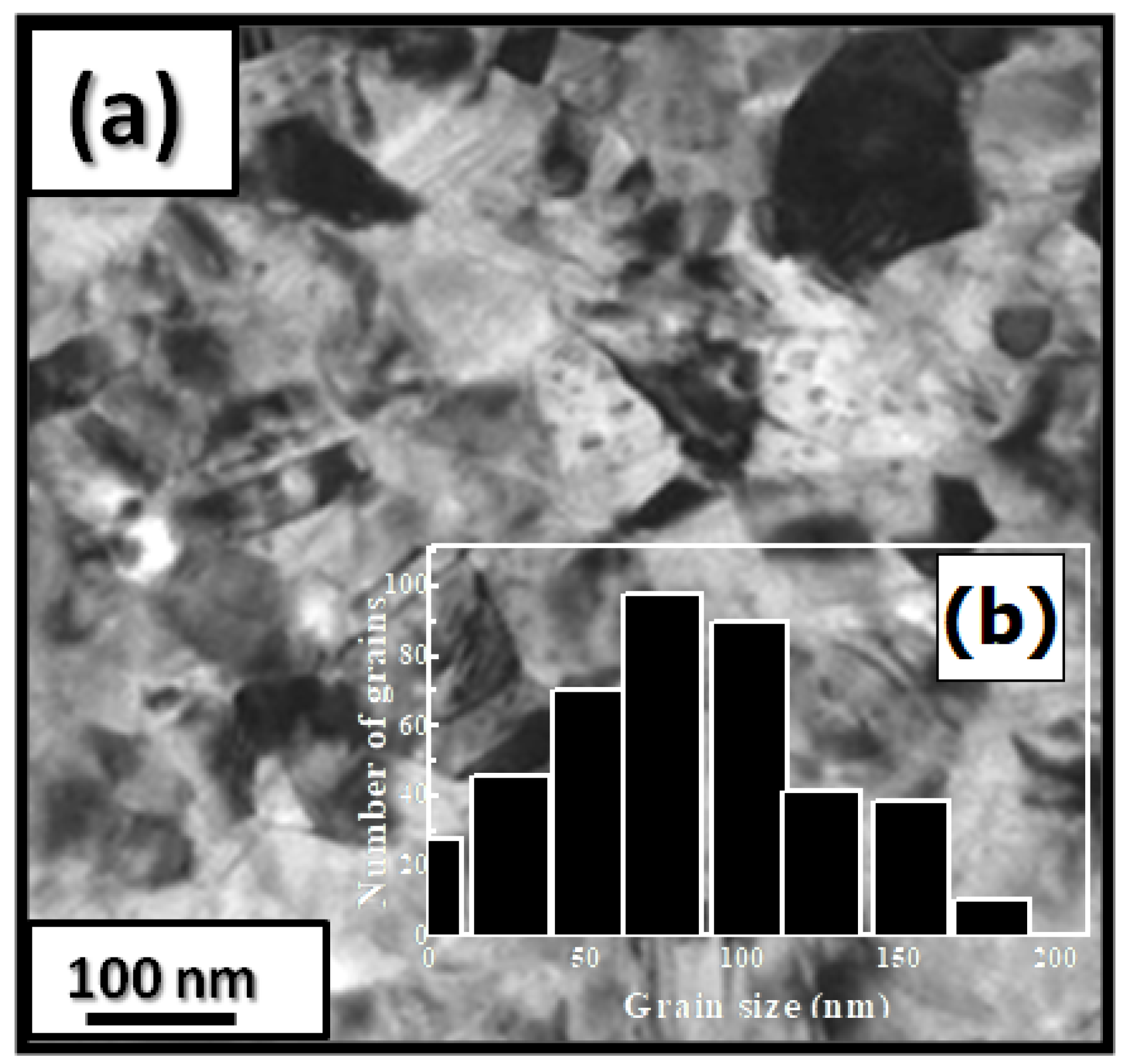
Figure 1. Bright-field micrography of the Pr2Co6.25Fe0.75 compound (a). The grain size distribution is shown in the inset (b).
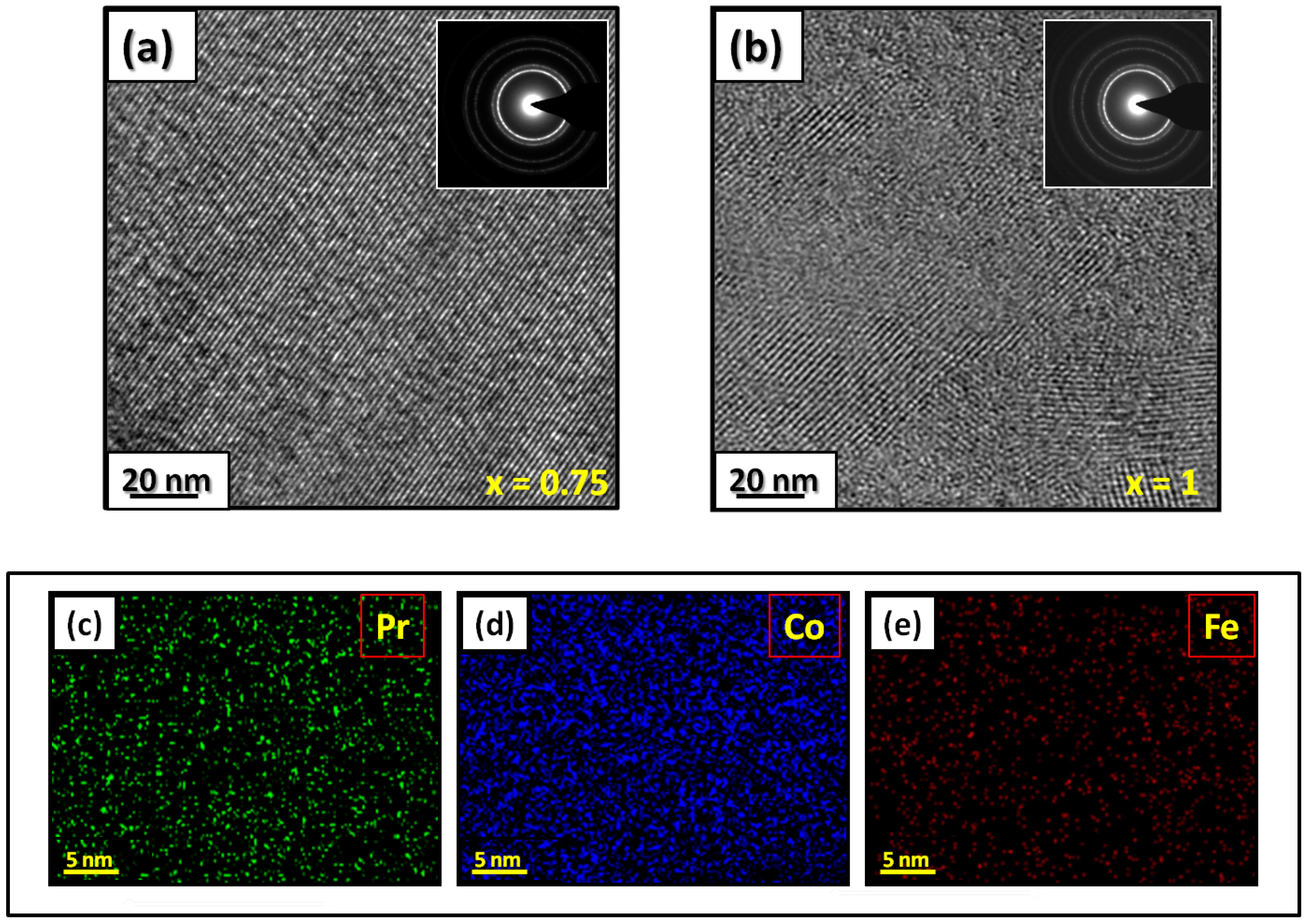
Figure 2. HRTEM images (the selected area of electron diffraction is shown in the inset): (a) Pr2Co6.25Fe0.75 and (b) Pr2Co6Fe1 compounds annealed at 1023 K. Elemental mapping of Pr2Co6.25Fe0.75: (c) Pr, (d) Co and (e) Fe.
4.3. Structural Analysis of Nanocrystalline Pr2Co7-xFex Compounds with EXAFS
Figure 3 displays the extended X-ray absorption fine structure (EXAFS) found for the Pr2Co7-xFex compounds (x = 0.5, 0.75, and 1). The Fe–K edge is near 7120 eV, with a maximum absorbance located at 7126 eV. The apparent features of the three spectra are similar, revealing that the local structure around the Fe atoms does not change significantly with Fe content. The Fourier transforms (FTs) of the EXAFS data (Figure 3) were calculated between k 2 Å and k = 10.5 Å − 1 in order to to avoid disturbance and noise. The resolution is /(2 k) = 0.18 Å. The main FT peak observed between 1.3 and 3.4 Å is due to the second atom shell (Pr and Fe) at ≈3.0 Å and the single Fe–Co scattering at ≈2.50 Å.
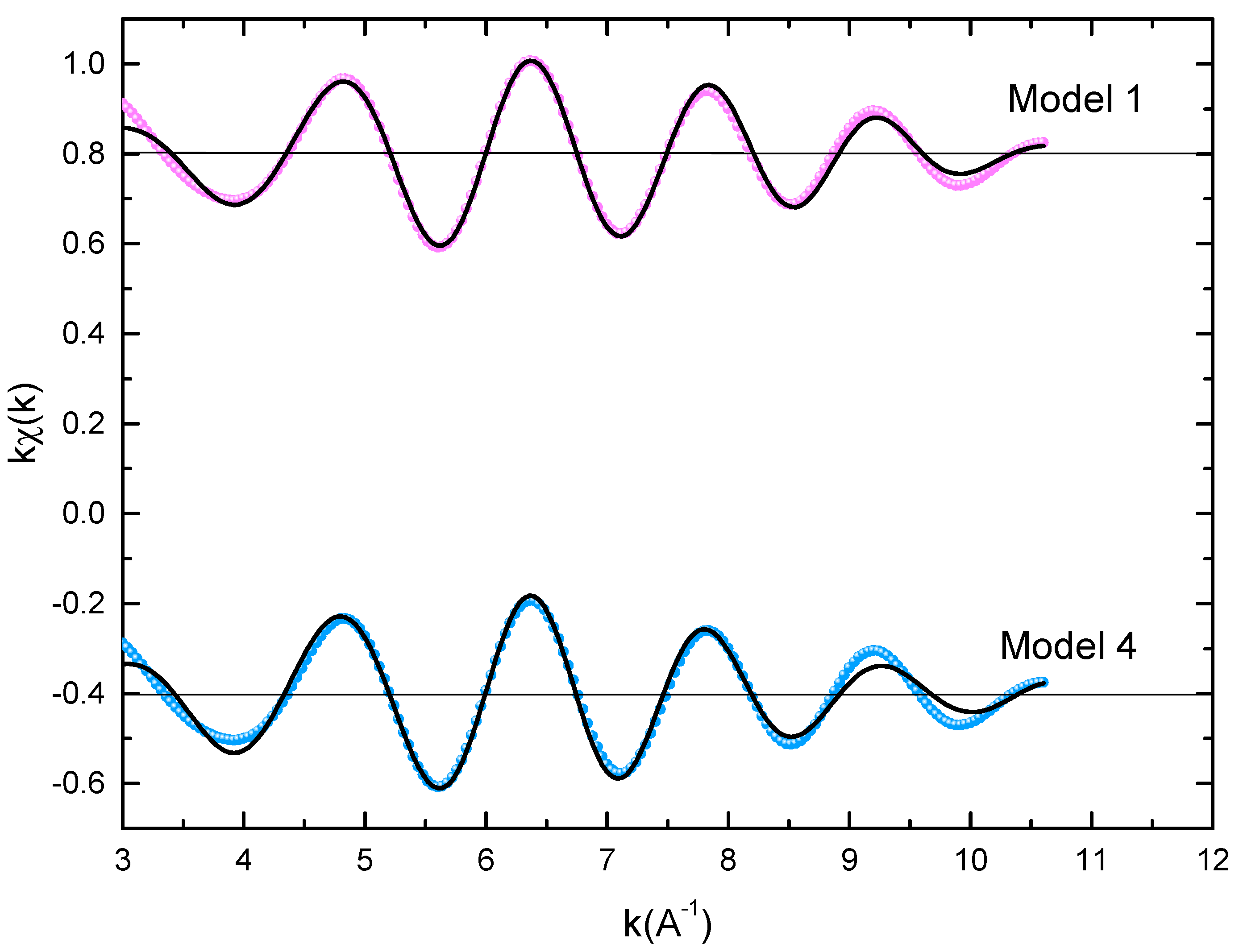
Figure 3. Experimental EXAFS spectra in k space of the imaginary part of the Fourier transforms of the Pr2Co6.5C0.5 compound, adjusted using Models 1 and 4. Black and red lines show the experimental signal and theoretical curves, respectively.
4.4. Nanocrystalline Pr2Co7Cx (x = 0–1) Compounds
Pr2Co7Cx compounds have a predominantly hexagonal Ce2Ni7 structure with a P63/mmc space group. The a and c parameters and the unit cell volume V are increased during C insertion ( a/a = 0.23%, c/c = 10.5% and V/V = 10%). Figure 4a shows a bright-field micrograph of the Pr2Co7C0.75 compound. The average grain size is around 150 nm (Figure 4b). The HRTEM images of the nanocrystalline Pr2Co7C0.75 and Pr2Co7C0.75 indicate a fully crystallized structure. The compositions of Pr, Co, and C are around 23.2, 74.8, and 2 atomic percent (at.%), respectively.
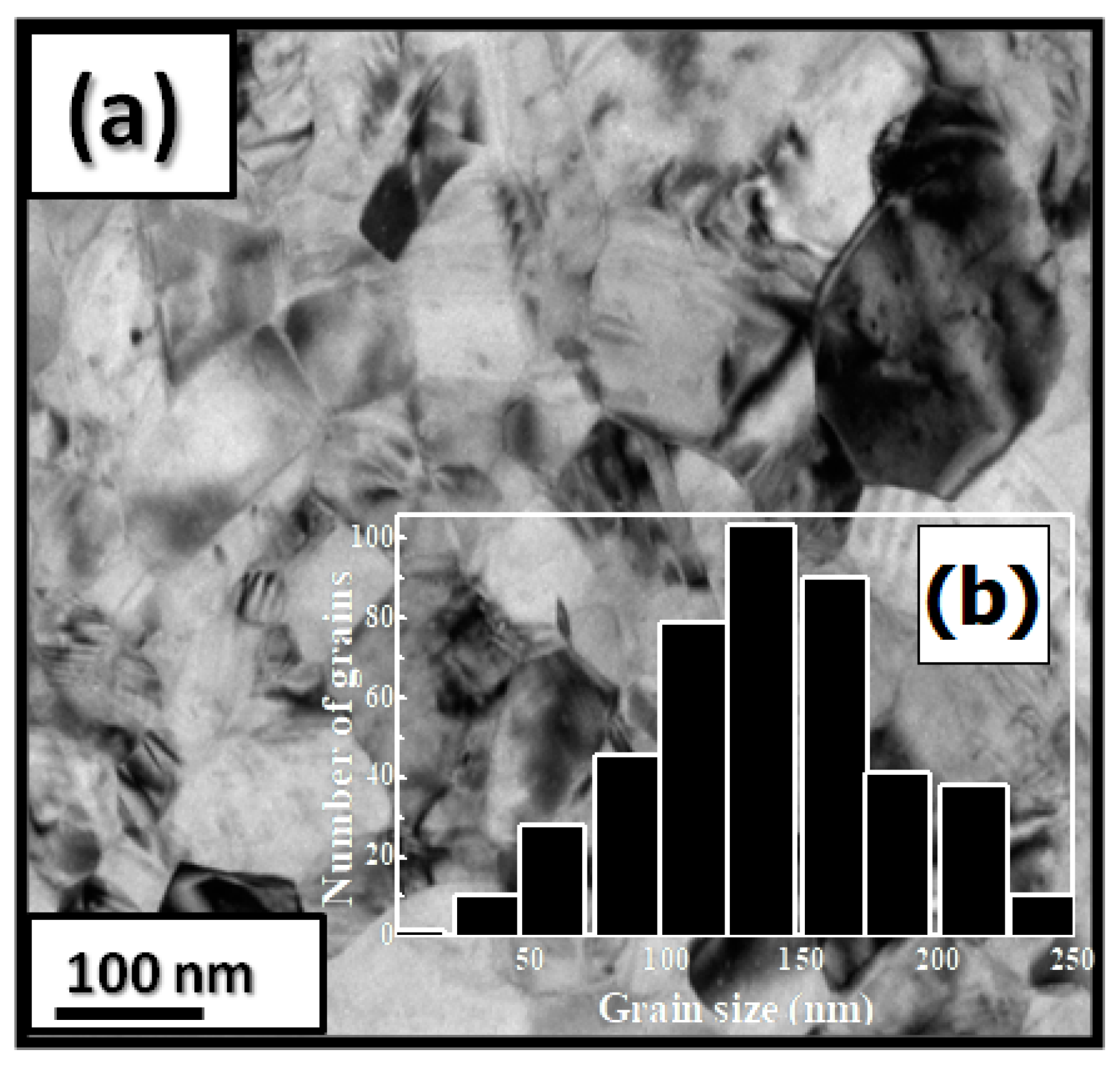
Figure 4. Bright-field micrographs of Pr 2 Co 7 C 0.75 (a). The grain size distribution is shown in the inset (b).
5. Intrinsic Magnetic Properties
5.1. Nanocrystalline Pr2Co7−xFex (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
To know the effects of Co substitution by Fe on the intrinsic magnetic properties of the alloy samples, the Curie temperature T for the nanocrystalline Pr2Co7−xFex phase was measured for each Fe content x. The nanocrystalline Pr2Co7−xFex compounds were ferromagnetic. The T C value increased from 600 K for the nanocrystalline Pr2Co7−x compound, attaining a maximum of 760 K at x = 0.5, and then decreased for x = 0.75 and x = 1. The T C values were around 695 and 667 K, respectively. The T C value was the result of two effects: the electronic effect related to the filling of the band of Fe atoms and the magnetovolume effect related to the Co-Co distances [68]. The M(H) curves of the nanocrystalline Pr2Co7−xFex compounds were measured at 293 K. The determination of was performed using the law of approach to saturation [71]:
The M S values were converted into the magnetic moment per formula unit .The μ s increased linearly with increasing the Fe content x. The theoretical equation of μ s as a function of x was defined by the formula: + 7x (0 ≤ x ≤ 1), where is the saturation moment of the Pr 2 Co 7 − x compound (in /f.u). The experimental data corresponded well to the theoretical forecast. The evolution of μ s with x could be justified by the reduction in the average number of 3d electrons when replacing the Co atoms with Fe, which led to a progressive strengthening of the Co(3d)-Co(3d) exchange interactions and, thus, to an enhanced magnetic moment with the 3d [68]. Scholars realized the XRD patterns of Pr 2 Co 7 − x Fe x powders that were magnetically aligned at T = 293 K in a field of 0.5 T. For samples with x < 0.5, the (00l) reflections were heavily enhanced, indicating EMD along the c axis. For x > 0.5, the diagram shows a lower intensity of the (00l) peaks, while the reflections (h0l) are reinforced, implying that the EMD of the Pr 2 Co 7 − x Fe x sample deviates from the c axis.
5.2. Nanocrystalline Pr2Co7Cx (x = 0–1) Compounds
The Curie temperature T C of the Pr 2 Co compounds was determined according to the Co-Co, Pr-Co, and Pr-Pr interactions. In general, the Co-Co interaction was dominant, and the Pr-Co and Pr-Pr interactions were negligible. From the obtained structural data, the C insertion led to a change in the a, c parameters of the Pr 2 Co 7 compound and thus to an increase in the unit cell volume V of 2.5%. The a, c parameters varied from Å and Å for Pr 2 Co 7 to Å and Å for Pr 2 Co 7 − x C. Consequently, the Co( )-Co( 12 k ), Co( 12 k )-Co( ), and Co( 4 f )-Co( 4 f ) distances increased the related , and exchange interactions. As a result, the T C increased due to the magnetovolume effect.
5.3. Nanocrystalline Pr2Co7Hx (x = 0–10.8) Hydrides
The H absorption resulted in a variation of the lattice parameters of the Pr 2 Co 7 compound and, therefore, an increase in the cell volume V. The nearest-neighbor number of each Co atom and the Co-Co distances were then determined depending on the H content. The Co-Co distances most influenced by H absorption were those involving the Co and Co atoms, respectively, in the 4f and 12k sites. Therefore, the increase in the Co 5 -Co 5 , Co 3 -Co 5 ,Co 3 -Co 3 , distances led to a modification of the exchange interactions J ,which had some impact on the increase in T C . T C increased as a function of H content from 600 K at x = 0 to 691 K at x = 3.75, then decreased for x > 3.75. The Pr 2 Co 7 H x hydrides presented a ferromagnetic comportment for all H contents. The XRD patterns of the magnetically aligned Pr 2 Co 7 H x hydride powder samples showed only (0 0 l)-type peaks, implying that the EMDs of Pr 2 Co 7 H x hydrides were along the c axis.
5.4. Mean Field Theory (MFT) and Random Magnetic Anisotropy (RMA) Analysis
5.4.1. Nanocrystalline Pr2Co7Hx (x = 0–1) Compounds
The ferromagnetic alignment of the magnetic moments of Pr and Co atoms, M and M , can be described using the following expression:
M P r and M C o were estimated using mean field theory (MFT). The magnetization M can be determined using expression (5). M C o and M P r were expected to follow the Brillouin functions [72]. M(T) was fitted for different C contents x. The experimental data align well with the theoretical curves. T C was found to be in agreement with the experimentally derived temperature. M P r , M C o and M were also determined for Pr 2 Co 7 C (Figure 5a). The J and J exchange interactions increased as a function of x, while J was found to be constant around 2.10 J. Inserting C into the Pr 2 Co 7 induced an enhancement of T C rom 600 to 730 K when x was increased from 0 to 1 (Figure 5b). The increase in T C can be ascribed to electronic and magnetovolumic effects.
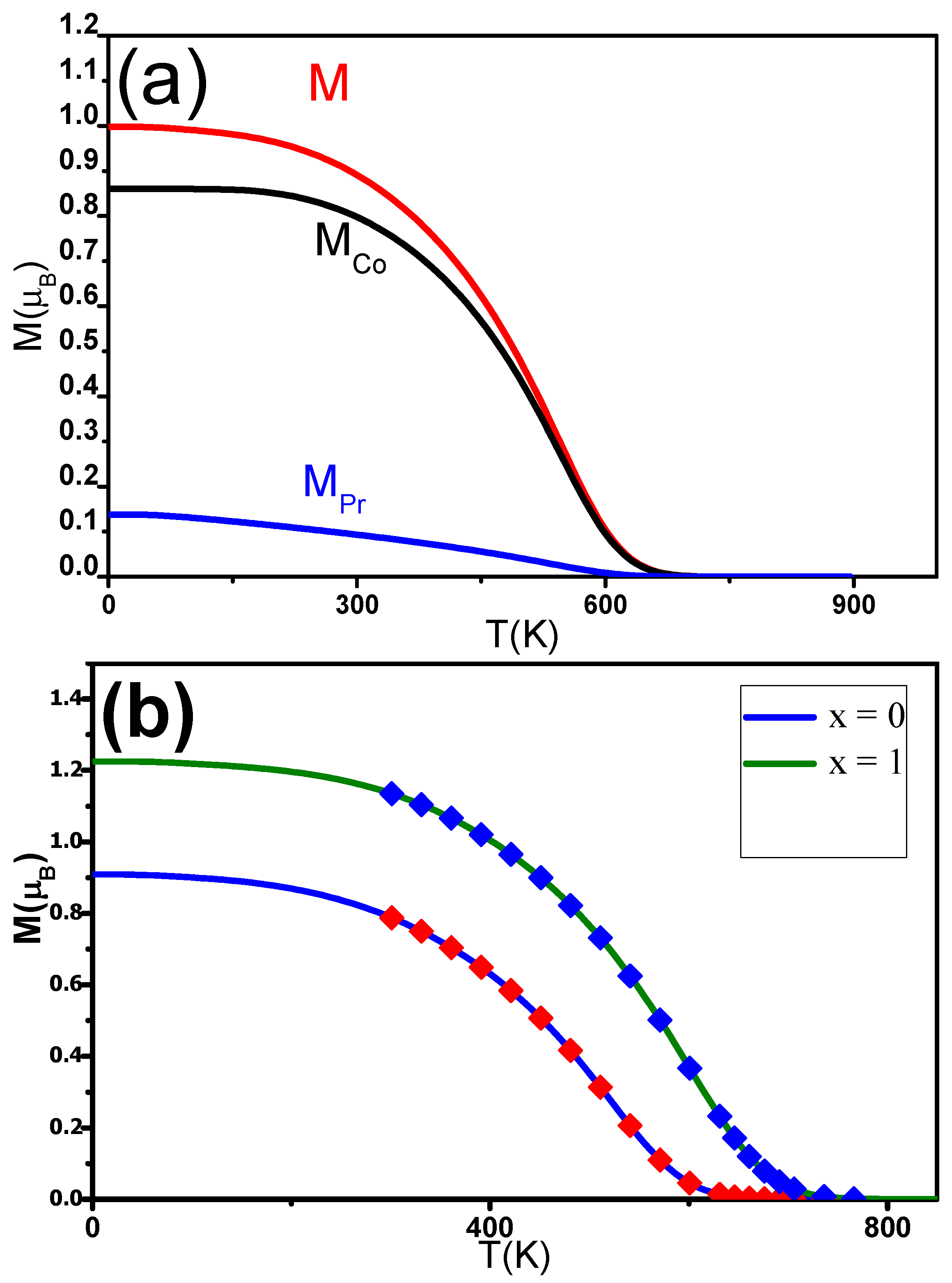
Figure 5. (a) Calculated change in the magnetic moments (M (T), M C o (T)) and magnetization (M(T)) of the nanocrystalline Pr2Co7C0.25 compound. (b) The solid lines are the M(T) of nanocrystalline Pr2Co7Hx found calculated in the MFT analysis. The symbols show the experimental data.
5.4.2. Nanocrystalline Pr2Co7Hx (x = 0–10.08) Compounds
The exchange interactions of nanocrystalline Pr 2 Co 7 H x were calculated as a function of H content using the MFT . J C o − C o and J C o − P r increased when the H content increased. The exchange interaction J was around 2 × 10 J. M (T), M (T), and M(T) were fitted. The T C values were found to be in good agreement with the experimentally determined values. T C increased as a function of H content from 600 K at x = 0 to 691 K at x = 3.75, dealing with an increase of about 9.1%, then decreasing for x > 3.75. The peak value appearance of T C at x = 3.75 resulted from the H content dependence of the exchange interaction parameter of J C o − C o . The enhancement of the T C of Pr 2 Co 7 H x hydrides for x ≤ 3.75 could be the result of two effects: the electronic effect associated with a decrease in the hybridization of Co(3d) atoms with their neighbors and the magnetovolume effect associated with Co-Co distances, which suggests a decrease in antiferromagnetic interaction [73]. The Co-Co exchange interactions increased from 166 × 10 J for x = 3.75. The decrease in T C for x > 3.75 was mostly due to the reduction in Co-Co exchange interactions from 178 × 10 − 23 to 130 × 10 − 23 J for x = 10.8.
5.5. Magnetocaloric Effect of the Nanocrystalline Pr2Co7 Compound
The measurements of the curves were carried out around the Curie temperature T C going from 562 to 598 K for 2:7 (R) and from 585 to 615 K for 2:7 (H). The same type of magnetization behavior as for Pr 2 Co 7 could be observed. On the other hand, when , the Pr 2 Co 7 compound was paramagnetic, and its magnetization increased gradually with the applied field. The magnetization increased rapidly with the applied field until saturation was achieved. It should be noticed that, for the 2:7 R structure, saturation was not achieved compared to the 2:7 H structure; this was related to the presence of strong uniaxial anisotropy in this compound. Scholars traced the Arrott curves for the Pr 2 Co 7 compound in its 2:7 H and 2:7 R structures based on the isotherms found. It should be remarked that these curves showed a positive slope; this confirmed that the Pr 2 Co 7 compound for the two structures had a second-order phase transition from the paramagnetic to the ferromagnetic state [74].
It calculated the evolution of the magnetic entropy as a function of temperature T and applied field H. Figure 6 and Figure 7 show the thermal variations in the changes in magnetic entropy for the 2:7 H and 2:7 R structures, respectively. The of the 2:7 R structure was greater than that for the 2:7 H structure. As shown in Figure 7, the maximum value was about 3.7 J/(kg.K) under a magnetic field of 0–15 kOe. There were two important contributions to the maximum change in magnetic entropy Δ S M : the M and , which was the difference between two successive isotherms at a particular magnetic field around the temperature T C . The peak that corresponded to Δ S M became a plateau starting at T C . This transition from a single peak to a plateau was seen for the first time for a second-order magnetic transition. The relative cooling power (RCP) values were 102.7 and 8.5 J/kg, respectively, for the 2:7 R and 2:7 H structures. The calculation was based on the variation of Δ S M according to the equation: [8], where Δ S M and are the maximum of the entropy variation and the full width at half maximum of the temperature dependence of the change in magnetic entropy Δ S M .
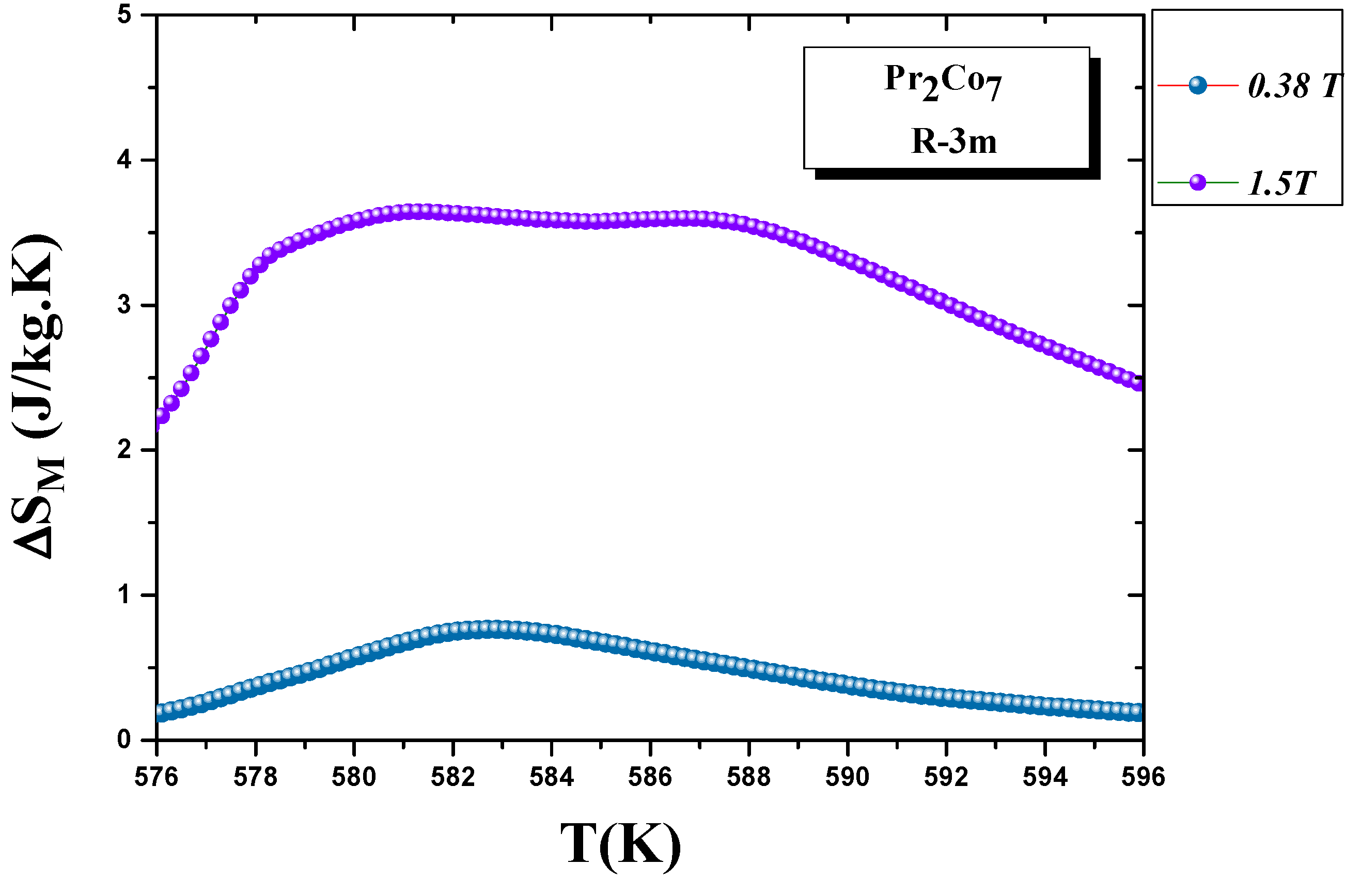
Figure 6. Magnetic entropy Δ S M (T) around the Curie temperature T C for the 2:7 H structure.
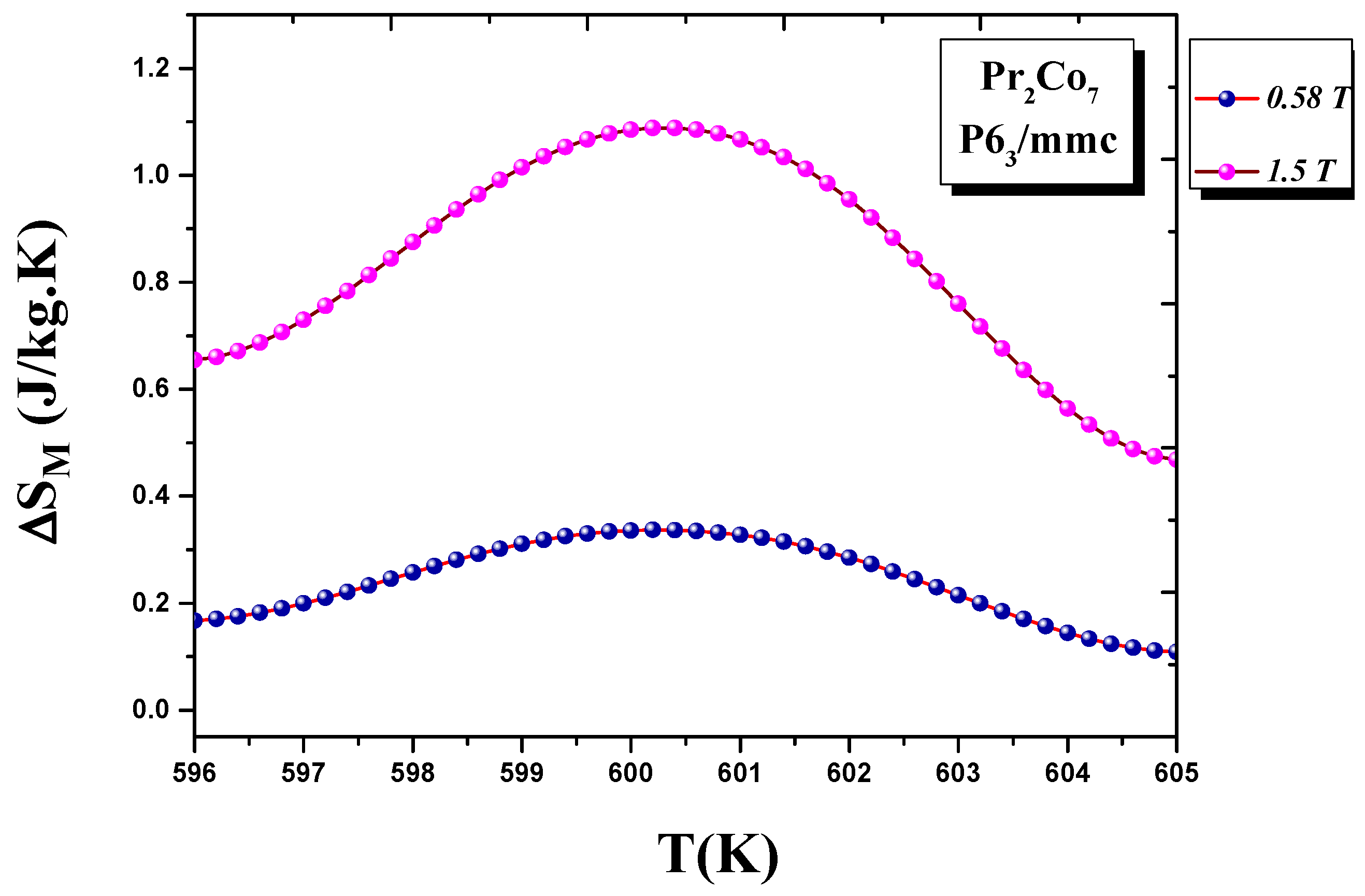
Figure 7. Magnetic entropy Δ S M (T) around the Curie temperature T C for the 2:7 R structure.
6. Extrinsic Magnetic Properties
6.1. Nanocrystalline Pr Co Fe (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
To obtain a nanocrystalline state with excellent extrinsic magnetic properties, annealing was performed at different temperatures. At each annealing temperature T , the hysteresis loops were measured. The evolution of Hc as a function of T a initially exhibited a characteristic increase in H with T a up to a maximum Hc c value, which was followed by a decrease in Hc c at higher temperatures of T a > 973 K. The most favorable microstructure for optimal magnetic properties corresponded to T a between 973 and 1073 K. The highest Hc c obtained for the nanocrystalline Pr Co Fe compounds was Hc c = 11.5 kOe and Hc c = 12 kOe, respectively. The respective values of remanent magnetization M were 43 and M r = 48 emu/g. The decrease in Hc c could mainly be due to the decrease in magnetocrystalline anisotropy after the substitution. Hc c depended principally on the magnetocrystalline anisotropy H a . The substitution of Co by Fe implied a reduction of the contribution of the axial and, thus, a decrease in Hc c as a function of x, as has been shown by X-ray diffraction in oriented powders [68]. Scholars studied the evolution of (BH) . The (BH) max value increased with T a and then decreases when T a increased for all values of Fe content x.
6.2. Nanocrystalline Pr 2 Co 7 H x (x = 0–1) Compounds
Figure 8 shows the hysteresis loops of the Pr 2 Co 7 , Pr 2 Co 7 C and Pr 2 Co 7 C compounds annealed at 1073 K and measured at 293 K. The hysteresis loops show that these compounds are isotropic and confirm that they have only one ferromagnetic phase. Scholars present in Figure 9 the evolution of H c as a function of annealing temperature T a for the Pr 2 Co 7 H x compounds. The curve passes through a maximum for = 1073 K. Two regions are thus highlighted; they are correlated to the mean grain size and the defects. The Hc c increases with T a in the lattice with the 2:7 H structure, i.e., with the grain size and the reduction of the defects, until the critical value found at 1073 K. The grains get bigger and Hc c decreases.
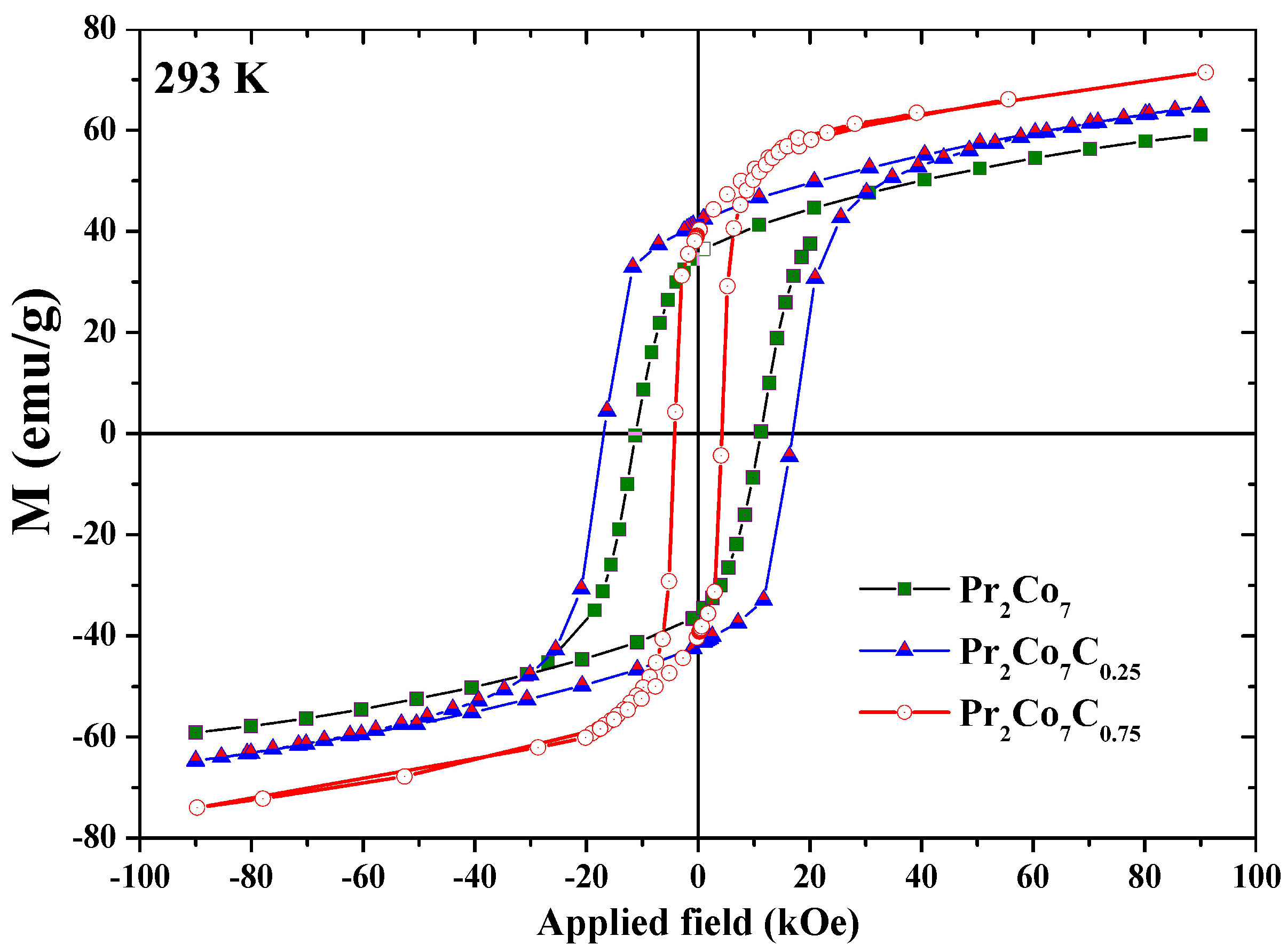
Figure 8. Hysteresis loops of the Pr 2 Co 7 , Pr 2 Co 7 C 0.25 and Pr 2 Co 7 C 0.75 compounds, measured at 293 K.
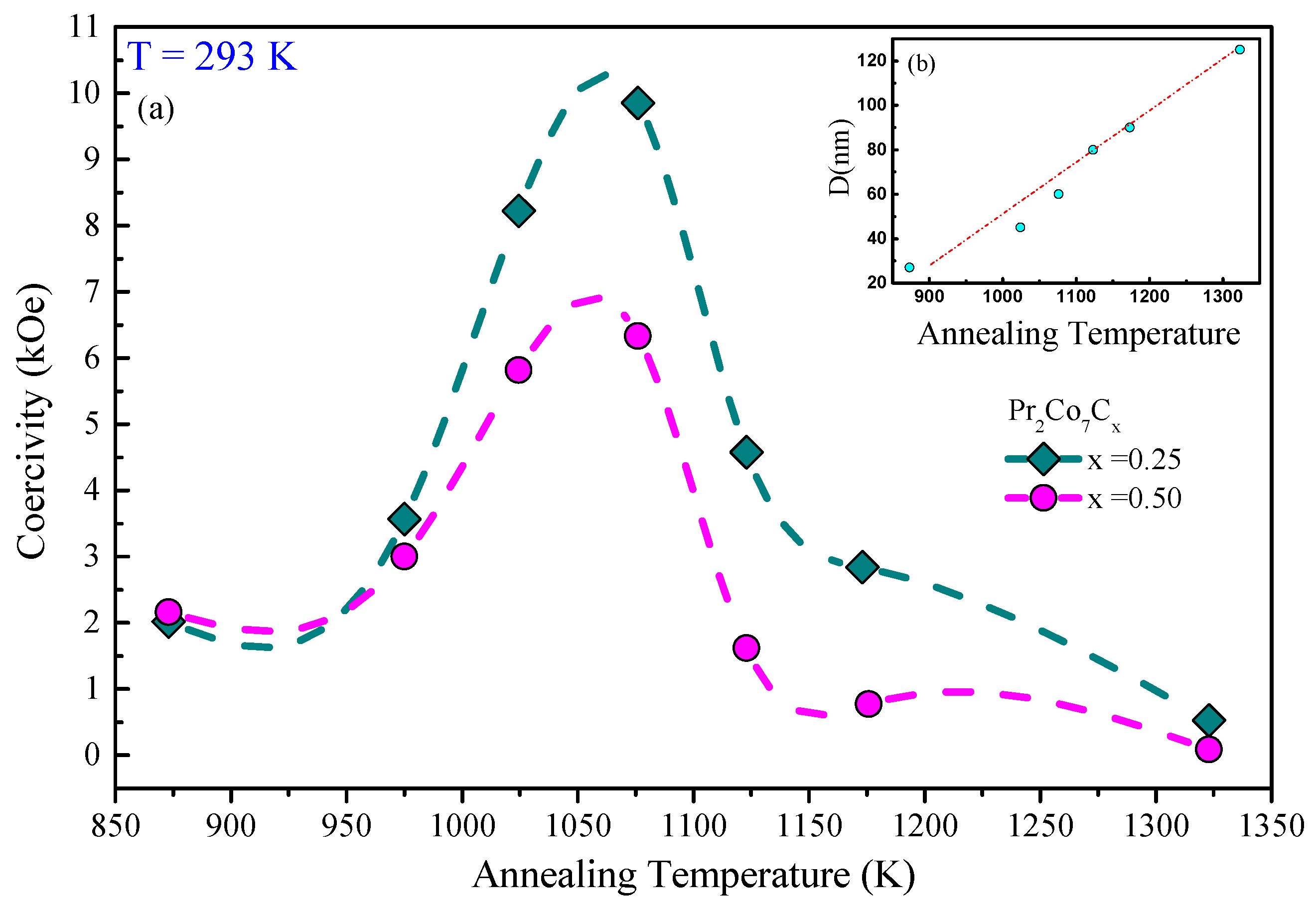
Figure 15. (a) The evolution of the coercivity H c as a function of annealing temperature T a of the Pr 2 Co 7 H x compounds ( ). (b) The grain size as a function of C content x is shown in the inset.
6.3. Nanocrystalline Pr 2 Co 7 H x (x = 0–10.8) Compounds
The demagnetization curves found in the hysteresis loops of the Pr 2 Co 7 H x hydrides (x = 0.25, 6.1, and 10.8) were smooth, indicating a fine and uniform grain size for these hydrides [73]. The Pr 2 Co 7 H 0.25 hydride had the highest extrinsic magnetic properties compared to the other Pr 2 Co 7 H x hydrides: Hc c = 6.1 KOe, (BH) max = 5.8 MGOe, M r = 40 emu/g, and remanence ratio M r /M max = 0.62. The Hc c decreased with x from about 11.5 KOe at x = 0 to 0.33 KOe at x = 10.8. The M r values ranged from 42.5 to 40.25 emu/g. The decrease in Hc c and (BH) max as a function of H content could mainly be due to the decrease in magnetocrystalline anisotropy resulting from the H absorption. The main interest lies in the fact that hydrogenation increased Tc c by a factor of almost 9.1% for x = 3.75 compared with the Pr 2 Co 7 compound. The Pr 2 Co 7 H 3.75 hydride could be considered as a soft material; it finds its applications in the field of magnetic recording or storage of information.
This entry is adapted from the peer-reviewed paper 10.3390/magnetochemistry8020020
References
- Burzo, E. Exchange Interactions and Transition Metal Moments in Rare-Earth Compounds. J. Synch. Investig. 2018, 12, 431–435.
- Zhang, Y. Review of the structural, magnetic and magnetocaloric properties in ternary rare earth RE2T2X type intermetallic compounds. J. Alloys Compd. 2019, 787, 1173–1186.
- Wang, Y.; Guo, D.; Wu, B.; Geng, S.; zhang, Y. Magnetocaloric effect and refrigeration performance in RE60Co20Ni20 (RE = Ho and Er) amorphous ribbons. J. Magn. Magn. Mater. 2020, 498, 166179.
- Li, L.; Yan, M. Recent progresses in exploring the rare earth based intermetallic compounds for cryogenic magnetic refrigeration. J. Alloys Compd. 2020, 823, 153810.
- Yin, L.; Parker, D.S. Effect of atom substitutions on the magnetic properties in Ce2Fe17: Toward permanent magnet applications. J. Appl. Phys. 2021, 129, 103902.
- Schafer, L.; Skokov, K.; Liu, J.; Maccari, F.; Braun, T.; Riegg, S.; Radulov, I.; Gassmann, J.; Merschroth, H.; Harbig, J.; et al. Design and Qualification of Pr-Fe-Cu-B Alloys for the Additive Manufacturing of Permanent Magnets. Adv. Funct. Mater. 2021, 31, 2102148.
- Dirba, I.; Sepehri-Amin, H.; Skokov, K.; Skourski, Y.; Hono, K.; Gutfleisch, O. Magnetic properties and microstructure of Sm5Fe17-based composite magnets. Acta Mater. 2021, 212, 116912.
- Jaballah, H.; Bouzidi, W.; Fersi, R.; Mliki, N.; Bessais, L. Structural, magnetic and magnetocaloric properties of (Pr,Sm)2Fe17 compound at room temperature. J. Phys. Chem. Solids 2022, 532, 110438.
- Chrobak, A.; Bajorek, A.; Chełkowska, G.; Haneczok, G.; Kwiencien, M. Magnetic properties and magnetocaloric effect of the Gd(Ni1−xFex)3 crystalline compound and powder. Phys. Status Solidi 2009, 206, 731–737.
- Bajorek, A.; Berger, C.; Pruzik, K.; Zubko, M.K.; Wojtyniak, M.; Chełkowska, G. Novel Ho(Ni0.8Co0.2)3 nanoflakes produced by high energy ball-milling. Mater. Charcterisation 2017, 128, 45–53.
- Lopadczak, P.; Bajorek, A.; Prusik, K.; Zubko, M.; Chełkowska, G. Magnetic hardening induced in RCo5 (R = Y, Gd, Sm) by short HEBM. Acta Phys. Pol. A 2018, 55, 2100904.
- Bajorek, A.; Łopadczak, P.; Prusik, K.; Zubko, M.; Chełkowska, G. The comparison of magnetic properties at room temperature in RCo5 (R = Y, Sm, Gd) nanoflakes synthesized via time-staged HEBM. IEEE Trans. Magn. 2019, 55, 2100904.
- Dahal, J.N.; Ali, K.S.S.; Mishra, S.R.; Neupane, D. Effect of Ga and Zr Substitution on the Properties of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx (0 ≤ x ≤ 1) Intermetallic Compounds Prepared via Arc Melting Process. Magnetochemistry 2020, 6, 9.
- Liu, K.; Wang, S.H.; Feng, Y.L.; Zhang, Y.K. Research on Phases and Morphology of Sm2Fe17 Melt-Spun Ribbon. Rare Met. Mater. Eng. 2020, 49, 3796–3802.
- Onoue, M.; Kobayashi, R.; Mitsui, Y.; Umetsu, R.Y.; Uwatoko, Y.; Koyama, K. Magnetic field-induced nitridation of Sm2Fe17. J. Alloys Compd. 2020, 835, 155193.
- Veselova, S.; Tereshina, I.; Verbetsky, V.; Neznakhin, D.; Tereshina-Chitrova, E.; Kaminskaya, T.; Karpenkov, A.; Akimova, O.; Gorbunov, D.; Savchenko, A. Structure and magnetic properties of (Sm,Ho)2Fe17Nx (x = 0; 2.4). J. Magn. Magn. Mater. 2020, 502, 166549.
- Kovacs, A.; Fischbacher, J.; Gusenbauer, M.; Oezelt, H.; Herper, H.C.; Vekilova, O.Y.; Nieves, P.; Arapan, S.; Schrefl, T. Computational Design of Rare-Earth Reduced Permanent Magnets. Engineering 2020, 6, 148–153.
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131.
- Bajorek, A.; Łopadczak, P.; Prusik, K.; Zubko, M. Correlation between Microstructure and Magnetism in Ball-Milled SmCo5/α-Fe (5%wt. α-Fe) Nanocomposite Magnets. Materials 2021, 14, 502.
- Ener, S.; Skokov, K.P.; Palanisamy, D.; Devillers, T.; Fischbacher, J.; Eslava, G.G.; Maccari, F.; Schafer, L.; Diop, L.V.B.; Radulov, I.; et al. Twins—A weak link in the magnetic hardening of ThMn12-type permanent magnets. Acta Mater. 2021, 214, 116968.
- Opelt, K.; Ahmad, T.; Diehl, O.; Schonfeldt, M.; Brouwer, E.; Vogel, I.; Rossa, J.D.; Gassmann, J.; Ener, S.; Gutfleisch, O. Upscaling the 2-Powder Method for the Manufacturing of Heavy Rare-Earth-Lean Sintered didymium-Based Magnets. Adv. Eng. Mater. 2021, 23, 2100459.
- Galler, A.; Ener, S.; Maccari, F.; Dirba, I.; Skokov, K.P.; Gutfleisch, O.; Biermann, S.; Pourovskii, L.V. Intrinsically weak magnetic anisotropy of cerium in potential hard-magnetic intermetallics. NPJ Quantum Mater. 2021, 2, 6.
- Hosokawa, A.; Suzuki, K.; Yamaguchi, W.; Takagi, K. Mechanism of anomalous α-Fe formation from stoichiometric Sm2Fe17 jet-milled powder during post-pulverization annealing. Acta Mater. 2021, 213, 116981.
- Shen, B.G.; Liang, B.; Wang, F.W.; Cheng, Z.H.; Gong, H.Y.; Zhang, S.Y.; Zhang, J.X. Magnetic Properties of Sm2Fe17−xSix and Sm2Fe17−xSixC Compounds. J. Appl. Phys. 1995, 77, 2637.
- Cao, L.; Handstein, A.; Gebel, B.; Schäfer, R.; Müller, K.H. Thermostability of Sm2(FeGa)17Cy prepared by gas-solid reaction (GSR). J. Appl. Phys. 1997, 81, 4539.
- van Lier, J.; Kubis, M.; Grünberger, W.; Shultz, L.; Kronmüller, H. High performance Sm2+δFe15Ga2C2 permanent magnets made by melt spinning and hot pressing. J. Appl. Phys. 1998, 83, 5549.
- Kubis, M.; Eckert, D.; Gebel, B.; Müller, K.H.; Schultz, L. Intrinsic magnetic properties of Sm2Fe17−xMxNy/Cy (M = Al, Ga or Si). J. Magn. Magn. Mater. 2000, 217, 14.
- Zheng, C.; Yu, D.; Li, K.; Luo, Y.; Jin, J.; Lu, S.; Li, H.; Mao, Y.; Quan, N. Effect of boron additions on phase formation and magnetic properties of TbCu7-type melt spun SmFe ribbons. J. Magn. Magn. Mater. 2016, 412, 89–94.
- Luo, Y.; Zhang, K.; Li, K.S.; Yu, D.B.; Ling, J.J.; Men, K.; Dou, Q.Y.; Yan, W.L.; Xie, J.J.; Yang, Y.F. Structure and magnetic behaviors of melt-spun SmFeSiB ribbons and their nitrides. J. Magn. Magn. Mater. 2018, 405, 214–218.
- Yang, W.; Zha, L.; Lai, Y.; Qiao, G.; Du, H.; Liu, S.; Wang, C.; Han, J.; Yang, Y.; Hou, Y.; et al. Structural and magnetic properties of the R10Fe90−xSix alloys with R=Y, Ce,Pr, Nd, Sm, Gd, Tb, Dy, Ho, and Er. Intermetallics 2018, 90, 8–17.
- Takagi, K.; Jinno, M.; Ozaki, K. Preparation of TbCu7-type Sm-Fe powders by low-temperature HDDR treatment. J. Magn. Magn. Mater. 2018, 454, 170–175.
- Wu, G.; Li, H.; Yu, D.; Li, K.; Yan, W.; Yuan, C.; Sun, L.; Luo, Y.; Zhang, K. Effect of niobium substitution on microstructures and thermalstability of TbCu7-type Sm-Fe-N magnets. J. Rare Earths 2018, 36, 281–286.
- Yan, W.; Quan, N.; Luo, Y.; Yu, D.; Wang, Z.; Wu, G.; Zhang, K. Structure and hard magnetic properties of TbCu7-type SmFe8.95−xGa0.26Nbx nitrides. J. Rare Earths 2018, 36, 165–169.
- Buschow, K.H.J. Magnetic anisotropy of some rare earth-cobalt compounds (R2Co7). J. Less-Common. Met. 1973, 33, 311.
- Givord, D.; Lemaire, R. Magnetic Tranition and Anomalous Thermal-Expansion in R2Fe17 Compounds. IEEE Trans. Magn. 1974, 10, 109–113.
- Buschow, K.H.J. Intermetallic compounds of rare-earth and 3d transition metals. Rep. Prog. Phys. 1977, 40, 1179.
- Kirchmayr, H.; Poldy, C. Magnetism in rare earth—3d intermetallics. J. Magn. Magn. Mater. 1978, 8, 1–42.
- Street, R.; Day, R.K.; Dunlop, J.B. Magnetic viscosity in NdFeB and SmCo5 alloys. J. Magn. Magn. Mater. 1987, 69, 106.
- Coehoorn, R.; Daalderop, G.H.O. Magnetocrystalline anisotropy in new magnetic materials. J. Magn. Magn. Mater. 1992, 104, 1081–1085.
- Qi, Q.; Li, Y.P.; Coey, J.M.D. Gas-phase interstitially modified intermetallics R(Fe11Ti)Z1-δ. II. 3d magnetization of the compounds Y(Fe11Ti)Z1-δ (Z = N, C). J. Phys. Condens. Matter 1992, 4, 8209.
- Fischer, R.; Kronmuller, H. Static computational micromagnetism of demagnetization processes in nanoscaled permanent magnets. Phys. Rev. B 1996, 54, 7284.
- Margarian, A.; Li, H.S.; Dunlop, J.B.; Cadogan, J.M. Structural and magnetic properties of the novel compound Dy3(Fe,Ti)29. J. Alloys Compd. 1996, 239, 27.
- Cataldo, L.; Lefevre, A.; Cohen-Adad, M. Elaboration et optimisation d’alliages magnetiques: Sm-Co-Cu-Fe-Zr. Etude par diagrammes de phases. J. Chim. Phys. 1997, A94, 1087–1093.
- Hu, J. Volume expansion and Curie temperature enhancement in R2Fe17Cx compounds. J. Alloys Compd. 1999, 285, 51.
- Fischer, R.; Kronmuller, H. The role of the exchange interaction in nanocrystalline isotropic Nd2Fe14B-magnets. J. Magn. Magn. Mater. 1999, 191, 225.
- Bessais, L.; Dorolti, E.; Djega-Mariadassou, C. Correlation between Sm2(Fe,Ga)17 and its precursor Sm(Fe,Ga)9. J. Appl. Phys. 2005, 97, 013902.
- Bessais, L.; Djega-Mariadassou, C.; Beaunier, P. Effect of nanocrystallization on the structure and the magnetic properties of Nd–Fe–Co–Al–B glassy alloy. J. App. Phys. 2006, 99, 093906.
- Yartys, V.; Riabov, A.; Denys, R.; Sato, M.; Delaplane, R. Novel intermetallic hydrides. J. Alloys Compd. 2006, 408, 273.
- Khazzan, S.; Mliki, N.; Bessais, L. Structure and magnetic properties of nanocrystalline Sm1-s(Fe,Mo)5+2s. J. Appl. Phys. 2009, 105, 103904.
- Bessais, L.; Younsi, K.; Khazzan, S.; Mliki, N. X-ray and intrinsic magnetic properties of nanocrystalline Sm2 (Fe, M) 17 (M = Si, Ga, Co, Cr, Zr or Mo). Intermetallics 2011, 19, 997–1004.
- Khazzan, S.; Mliki, N.; Bessais, L.; Djega-Mariadassou, C. Rare-earth iron-based intermetallic compounds and their carbides: Structure and magnetic behaviors. J. Magn. Magn. Mater. 2010, 322, 224–229.
- Kowalczyk, A. The intrinsic magnetic properties of R2Co7B3 (R= rare earth) intermetallic compounds. J. Magn. Magn. Mater. 1997, 175, 279.
- Apostolov, A.; Bozukov, L.; Stanev, N.; Mydlar, T. A change in magnetic properties of R2Co7 intermetallic compounds (R = Pr, Sm, Tb and Ho) upon hydrogen absorption. J. Magn. Magn. Mater. 1990, 83, 286.
- Fersi, R.; Mliki, N.; Bessais, L.; Guetari, R.; Russier, V.; Cabie, M. Effect of annealing on structural and magnetic properties of Pr2Co7 compounds. J. Alloys Compd. 2012, 14, 522.
- Deryagin, A.V. 3d-4f METALLIC COMPOUNDS. Magnetic moment, magnetic anisotropy and spin-reorientation phase transition in (4f-3d)-intermetallic compounds. J. Phys. 1979, C5, 165.
- Dunlap, R.; Small, D.; MacKay, G.; O’Brien, J.W.; Dahn, J.R.; Cheng, Z.H. Materials preparation by ball milling. J. Can. Phys 2000, 78, 211.
- Fersi, R.; Cabie, M.; Mliki, N.; Bessais, L. Impact of carbon insertion on the microstructure and magnetic properties of nanocrystalline Pr2Co7 alloys. J. Alloys Compd. 2013, 576, 415–423.
- Yamkane, Z.; Fersi, R.; Rachid, F.; Moubah, R.; Lassri, H.; Mliki, N.; Bessais, L. Random magnetic anisotropy studies in nanocrystalline Pr2Co7Hx (0 < x < 3.75) hydrides. J. Magn. Magn. Mater. 2018, 449, 461–466.
- Gal, L.; Charbonnier, V.; Zhang, J.; Goubault, L.; Bernard, P.; Latroche, M. Optimization of the La substitution by Mg in the La2Ni7 hydride-forming system for use as negative electrode in Ni-MH battery. Int. J. Hydrogen Energy 2015, 40, 17017–17020.
- Fersi, R.; Cabie, M.; Mliki, N.; Bessais, L. Effect of annealing temperature on the microstructure of Pr2Co7 alloys and its hydrogen aborption-desorption kinetics. Int. J. Hydrogen Energy 2019, 44, 22011–22021.
- Rosetti, I.; Ramis, G. Quantification of delivered H2 by a volumetric method to test H2 storage materials. Int J Hydrogen Energy 2013, 38, 13309.
- Zhang, J.; Charbonnier, V.; Madern, N.; Monnier, J.; Latroche, M. Improvement of reversible H storage capacity by fine tuning of the composition in the pseudo-binary systems A2−xLaxNi7 (A = Gd, Sm, Y, Mg). J. Alloys Compd. 2021, 852, 157008.
- Rietveld, H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152.
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71.
- Michalowicz, A.; Moscovici, J.; Muller-Bouvet, D.; Provost, K. Max: Multiplatform applications for xafs. J. Phys. Conf. Ser. 2009, 190, 012034.
- Bez, R.; Fersi, R.; Zehani, K.; Moscovici, J.; Bessais, L.; Mliki, N.; Fonda, E.; Michalowicz, A. Phase stability, EXAFS investigation and correlation between nanostructure and extrinsic magnetic properties of nanocrystalline Pr2(Co,Fe)7. J. Alloys Compd. 2016, 666, 317.
- Ankudinov, A.; Ravel, B.; Rehr, J.; Conradson, S. Real-space multiplescattering calculation and interpretation of x-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565.
- Fersi, R.; Mliki, N.; Cabié, M.; Bessais, L. Intrinsic and extrinsic magnetic properties of nanocrystalline Pr2(Co,Fe)7. Phys. Status Solidi 2014, 211, 910.
- Charbonnier, V.; Zhang, J.; Monnier, J.; Goubault, L.; Bernard, P.; Magén, C.; Serin, V.; Latroche, M. Structural and Hydrogen Storage Properties of Y2Ni7 Deuterides Studied by Neutron Powder Diffraction. J. Phys. Chem. C 2015, 119, 12218–12225.
- Paul-Boncour, V.; Crivello, J.; Madern, N.; Zhang, J.; Diop, L.; Charbonnier, V.; Monnier, J.; Latroche, M. Correlations between stacked structures and weak itinerant magnetic properties of La2-xYxNi7 compounds. J. Phys. Condens. Matter 2020, 32, 415804.
- Néel, L. Relation entre la constante d’anisotropie et la loi d’approche à la saturation des ferromagnétiques. Rep. Prog. Phys. 1948, 9, 148.
- Masrour, R.; Jabar, A.; Khlif, H.; Jemaa, F.B.; Ellouze, M.; Hlil, E.K. Experiment, mean field theory and Monte Carlo simulations of the magnetocaloric effect in La0.67Ba0.22Sr0.11MnO3 compound. Solid State Commun. 2017, 268, 64.
- Fersi, R.; Mliki, N.; Bessais, L. Hydrogenation and the effect of H absorption on structural and magnetic properties of nanocrystalline Pr2Co7 alloys. J. Magn. Magn. Mater. 2018, 465, 220–227.
- Bouzidi, W.; Mliki, N.; Bessais, L. Second-order magnetic transition and low field magnetocaloric effect in nanocrystalline Pr5Co19 compound. J. Electron. Mater. 2018, 47, 2776–2781.
