The Pr2Co7 compound has interesting magnetic properties, such as a high Curie temperature TC and uniaxial magnetocrystalline anisotropy. It crystallizes in a hexagonal structure (2:7 H) of the Ce2Ni7 type and is stable at relatively low temperatures (Ta ≤ 1023 K), or it has a rhombohedral structure (2:7 R) of the Gd2Co7 type and is stable at high temperatures (Ta ≥ 1223 K). Studies of the magnetocaloric properties of the nanocrystalline Pr2Co7 compound have shown the existence of a large reversible magnetic entropy change (ΔSM) with a second-order magnetic transition.
- magnetic properties
- magnetocaloric properties
- Pr2Co7 Compound
1. Introduction
In recent years, magnetic nanomaterials based on rare-earth elements (R) and transition metals (T) have been widely investigated due to their extremely diverse potential applications in industrial fields [1,2,3,4,5,6,7,8]. These properties are often used to produce soft, hard, or semi-hard magnetic materials [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The origin of these exceptional magnetic properties is particularly due to the coexistence of of two complementary kinds of magnetism: the localized magnetism characteristic of rare-earth (R) electrons and the itinerant magnetism of the electrons of transition metals (T), such as cobalt (Co) and iron (Fe) [24,25,26,27,28,29,30,31,32,33]. The R elements thus provide their strong magnetocrystalline anisotropy (H ) due to the interactions between their orbital moment and the crystal field. The metals provide their strong magnetization and a high Curie temperature (T ) due to the important exchange interactions between the elements [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Permanent magnets are the idea of an ever-increasing number of recent devices. Alloys and intermetallic compounds obtained by combining (R) elements with metals (T) form a crucial class of materials for which applications have been found in permanent magnets [44]. Among the intermetallic systems, the noncrystalline Pr Co compound is currently one of the most promising [34,52,53,54]. The interest in these systems is due to the combination of the complementary characteristics of the -itinerant and -localized magnetism of Co and Pr atoms, respectively [54,55]. In order to strengthen these interactions, it is necessary to partially substitute the Co atoms in the noncrystalline Pr Co compounds with an appropriate element, such as iron (Fe), or through the insertion of a light element, such as carbon (C) or hydrogen (H), which can increase interatomic distances and strengthen the magnetic moment.
2. Synthesis Methods of Pr Co , Pr Co Fe , Pr Co C ,and Pr Co H Samples
To prepare the nanocrystalline Pr Co and Pr Co Fe (x = 0.25, 0.5, 0.75, and 1) samples, high-energy ball milling was carried out on previously prepared ingots [60]. The pre-alloy obtained by fusion was broken inside a glove box with a mortar and then introduced with five beads into a hermetically sealed jar. A Fritsch P7 planetary mill with a bead/powder ratio of 15:1 was used to get finer particles. The ground powder was then wrapped in tantalum foil (Ta) and then sealed in quartz ampoules under secondary vacuum (10 bar) at different annealing temperatures: T = 700 K and 1350 K. The tantalum foil (Ta) was used to avoid contamination due to contact with silica; these samples were first degassed under secondary vacuum to ensure that no impurities came to pollute the synthesis products. The sample was cooled after leaving the oven by quenching in water. To insert carbon atoms (C) into the Pr Co elemental cell, we used a carbonation technique that involved a solid–solid-type reaction according to the following reaction [61]:
Hydrogen atoms (H) were inserted into the Pr Co compound with a solid–gas reaction between the sample and 99.99% pure dihydrogen (H ) [58]. The method used was that of Sievert [62,63]. The amount of absorbed hydrogen was determined with a volumetric method [64,65].
3. Structural, Microstructural, and Magnetic Characterizations of the Samples
The microstructural characterizations of the Pr Co , Pr Co Fe , Pr Co C , and Pr Co H samples were investigated by using X-ray diffraction (XRD; Bruker D8 Advance) with CuK radiation of wavelength = 0.154056 nm. The refinement of the patterns was done by using the FULLPROF computing code based on the Rietveld method [66,67] with the assumption of the Thompson–Cox–Hastings line profile. The goodness-of-fit indicators and R were calculated as previously described in [61]. Extended X-ray absorption fine-structure (EXAFS) measurements were performed on a 2.75 GeV SAMBA beamline, Synchrotron SOLEIL, France. EXAFS experiments were carried out at 293 K in the fluorescence mode using a 4-element Si(111) drift detector. The EXAFS spectra were extrapolated using the MAXeCherokee code [68,69], while the fitting process and comparison between theoretical and experimental EXAFS curves were carried out with the MAX-Roundmidnight package [68]. The theoretical phases and amplitudes used in the EXAFS models were determined with the FEFF8 code [70] by using the crystal structure results of the Rietveld refinements at each Fe site with the use of the MAX-CRYSTALFFREV code [68] for the Crystal Structure–EXAFS Modeling interface.
The morphology, the chemical compositions, and the images were considered using a JEOL 2010 FEG transmission electron microscope equipped with an energy-dispersive spectrometer (EDS). For the TEM measurements, specimens were thinned using a focused-ion-beam-type FEI Helios 600 Nanolab dual-beam instrument. The Curie temperature T was measured on a MANICS differential sample magnetometer (DSM-871 Magneto/susceptometer) in a field of 1 kOe with a sample of around 5–10 × 10 g. T was obtained from the M(T) curve by extracting the linear part of the M(T) curve and determining the temperature value of the intersection with the expanded baseline. Magnetic hysteresis M(H) loops were determined using a Physical Properties Measurement System (PPMS9) Quantum Design microscope.
4. Structural Properties
4.1. Nanocrystalline Pr Co Compound
The nanocrystalline Pr Co compound can crystallize into two polymorphic structures [54] as a function of the annealing temperature T ; the first is a hexagonal (2:7 H) structure of the Ce Ni type ( space group) that is stable at T ≤ 1023 K with the lattice parameters Å and Å. The second is a rhombohedral (2:7 R) structure of the Gd Co type ( space group) that is stable at Ta ≥ 1223 K. The lattice parameters are Å and c = 36.549 ÅṪhe Pr Co cells can be defined by stacking the hexagonal structural blocks for PrCo (CaCu -type structure) and the cubic PrCo blocks (MgCu - and MgZn -type structures) along the common hexagonal (trigonal for PrCo ) axis [54]. The lattice parameters of the two structures are distinguished by the c parameter, which is higher for the 2:7 R structure due to the difference in stacking ([2H] = [BBA BBA ], [3R ] = 3[BBA ]) [71,72,73].
4.2. Nanocrystalline Pr Co Fe (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
Figure 1 presents bright-field micrographs of the Pr Co Fe (Figure 1a) compound. By performing statistical calculations on the grain size, the grain size distributions are shown in Figure 1b. The grain size was estimated to be 90 nm. The values found are in good agreement with the average grain sizes found using Scherrer’s expression. The HRTEM images shown in Figure 2a,b reveal the fully crystallized structure of the Pr Co Fe and Pr Co Fe compounds. The composition distribution was investigated by STEM–EDX mapping (Figure 2c–e. The stoichiometric proportions of Pr Co Fe were maintained. The Pr, Co, and Fe compositions ranged from 20.8 to 23.5%, 78.9 to 75.5%, and 1.4 to 2.6%, respectively. The diffraction spots were distributed over the rings corresponding to the d (0.795 nm), d (0.635 nm), d (0.508 nm), d (0.458 nm), d (0.291 nm), d (0.258 nm), and d (0.158 nm) distances for the (2:7 H) structure [69]
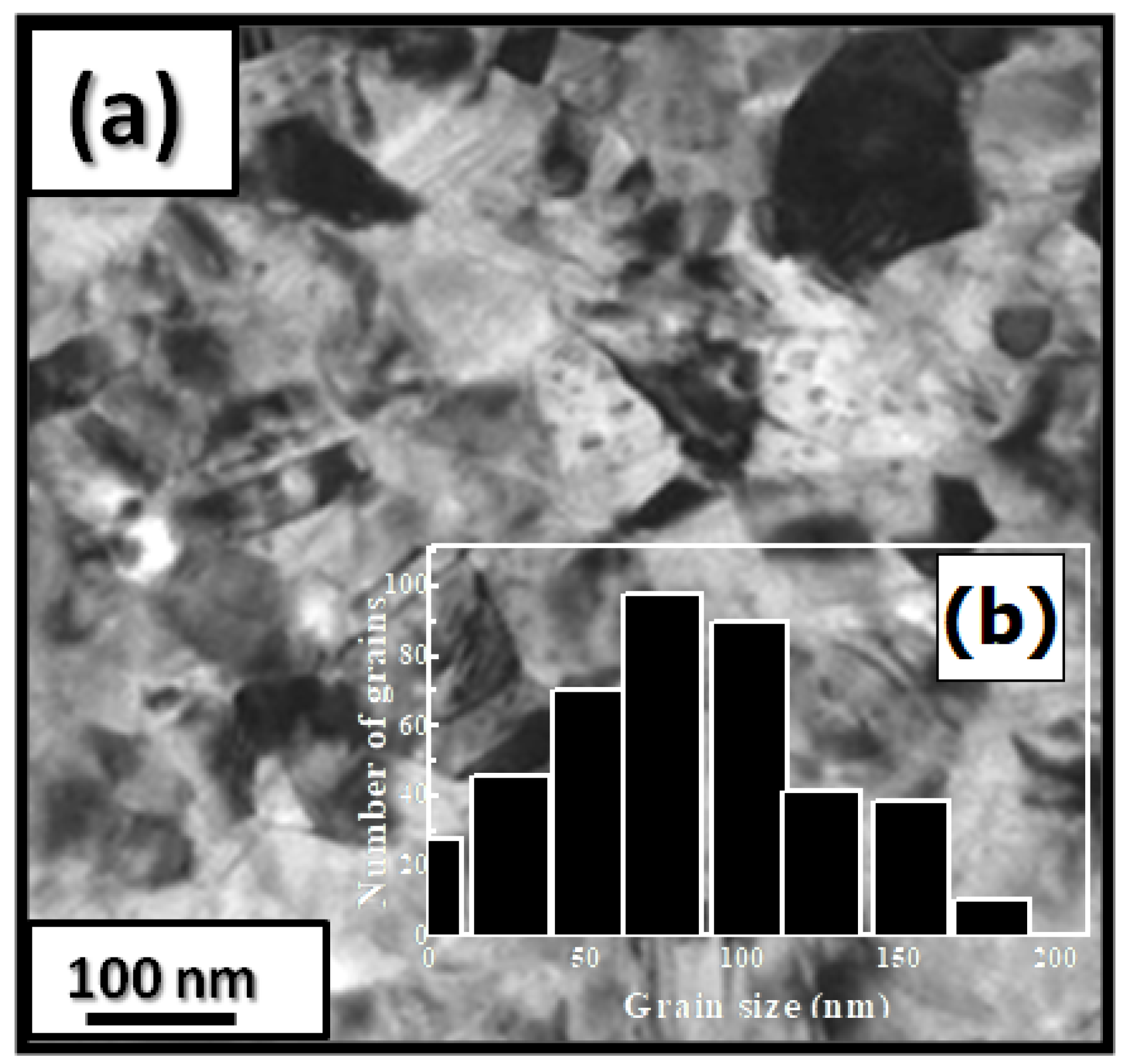
Figure 1. Bright-field micrography of the Pr Co 6.25 Fe 0.75 compound (a). The grain size distribution is shown in the inset (b).
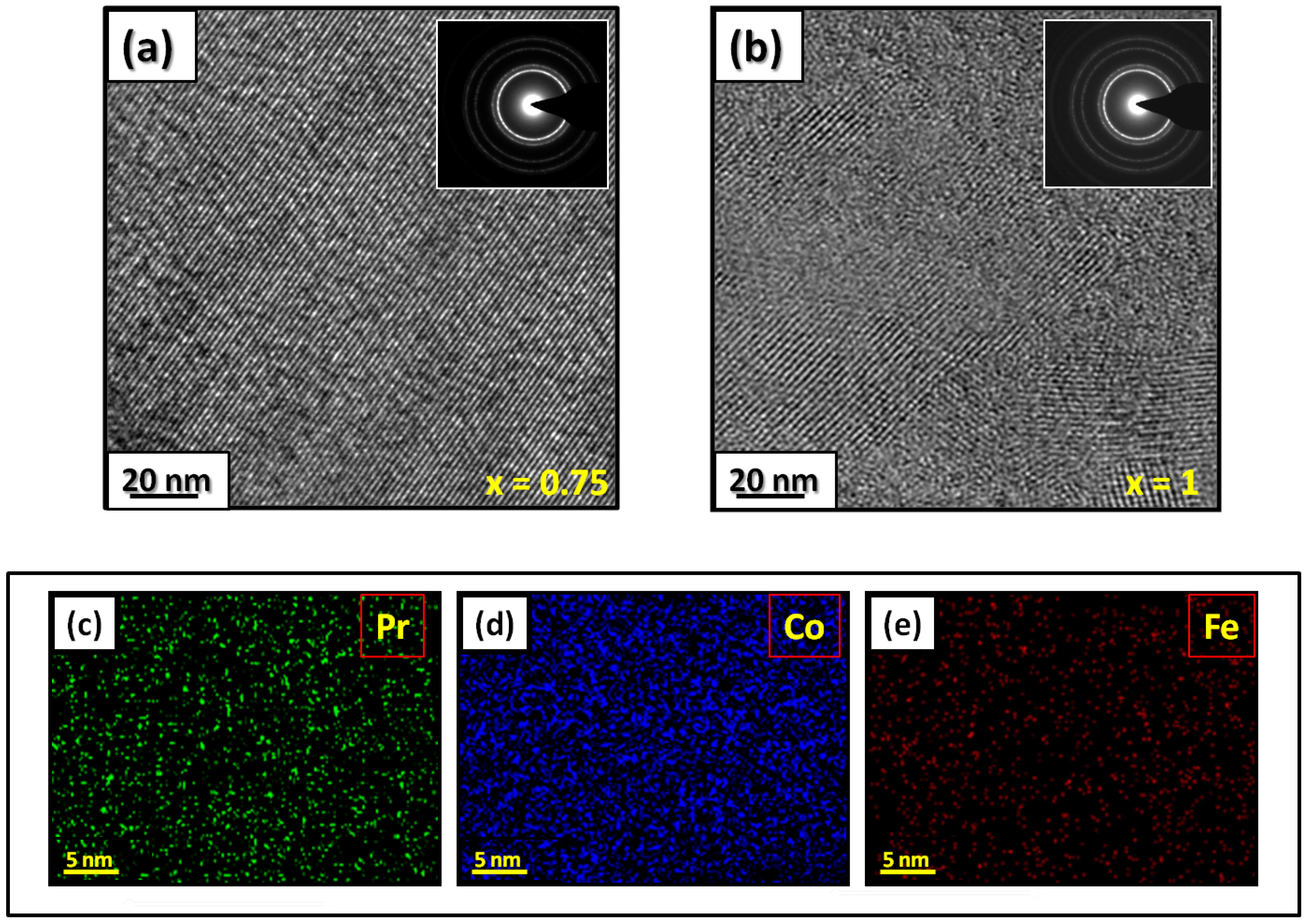
Figure 2. HRTEM images (the selected area of electron diffraction is shown in the inset): (a) Pr 2 Co 6.25 Fe 0.75 and (b) Pr Co Fe compounds annealed at 1023 K. Elemental mapping of Pr 2 Co 6.25 Fe 0.75 : (c) Pr, (d) Co and (e) Fe.
4.3. Structural Analysis of Nanocrystalline Pr 2 Co 7 − x Fe x Compounds with EXAFS
Figure 3 displays the extended X-ray absorption fine structure (EXAFS) found for the Pr Co Fe compounds (x = 0.5, 0.75, and 1). The Fe–K edge is near 7120 eV, with a maximum absorbance located at 7126 eV. The apparent features of the three spectra are similar, revealing that the local structure around the Fe atoms does not change significantly with Fe content. The Fourier transforms (FTs) of the EXAFS data (Figure 3) were calculated between k 2 Å and k = 10.5 Å − 1 in order to to avoid disturbance and noise. The resolution is /(2 k) = 0.18 Å. The main FT peak observed between 1.3 and 3.4 Å is due to the second atom shell (Pr and Fe) at ≈3.0 Å and the single Fe–Co scattering at ≈2.50 Å.
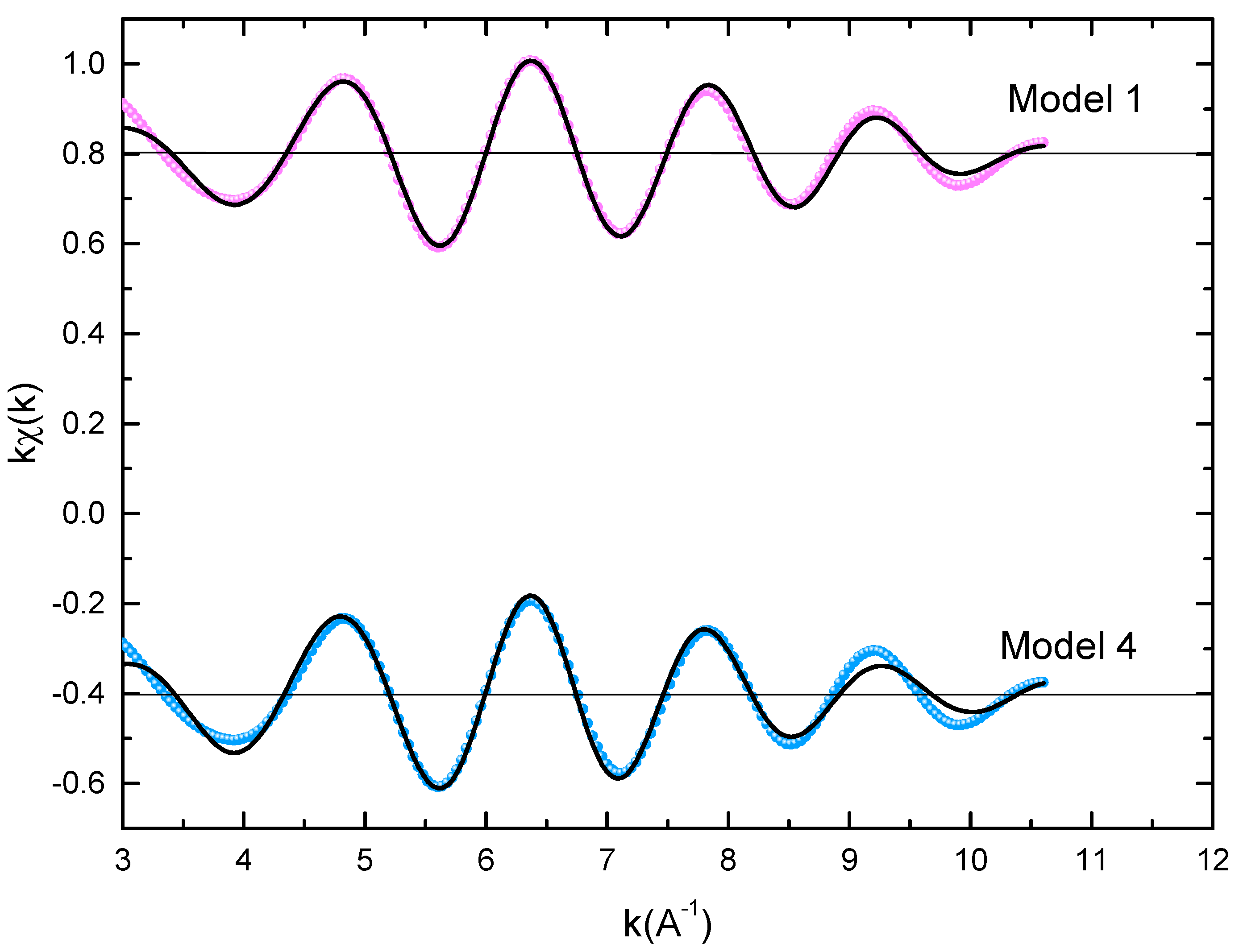
Figure 3. Experimental EXAFS spectra in k space of the imaginary part of the Fourier transforms of the Pr Pr Fe compound, adjusted using Models 1 and 4. Black and red lines show the experimental signal and theoretical curves, respectively.
4.4. Nanocrystalline Pr Co (x = 0–1) Compounds
Pr Co C compounds have a predominantly hexagonal Ce Ni structure with a P63/mmc space group. The a and c parameters and the unit cell volume V are increased during C insertion ( a/a = 0.23%, c/c = 10.5% and V/V = 10%). Figure 4a shows a bright-field micrograph of the Pr 2 Co 7 C compound. The average grain size is around 150 nm (Figure 4b). The HRTEM images of the nanocrystalline Pr 2 Co 7 C and Pr 2 Co 7 C indicate a fully crystallized structure. The compositions of Pr, Co, and C are around 23.2, 74.8, and 2 atomic percent (at.%), respectively.
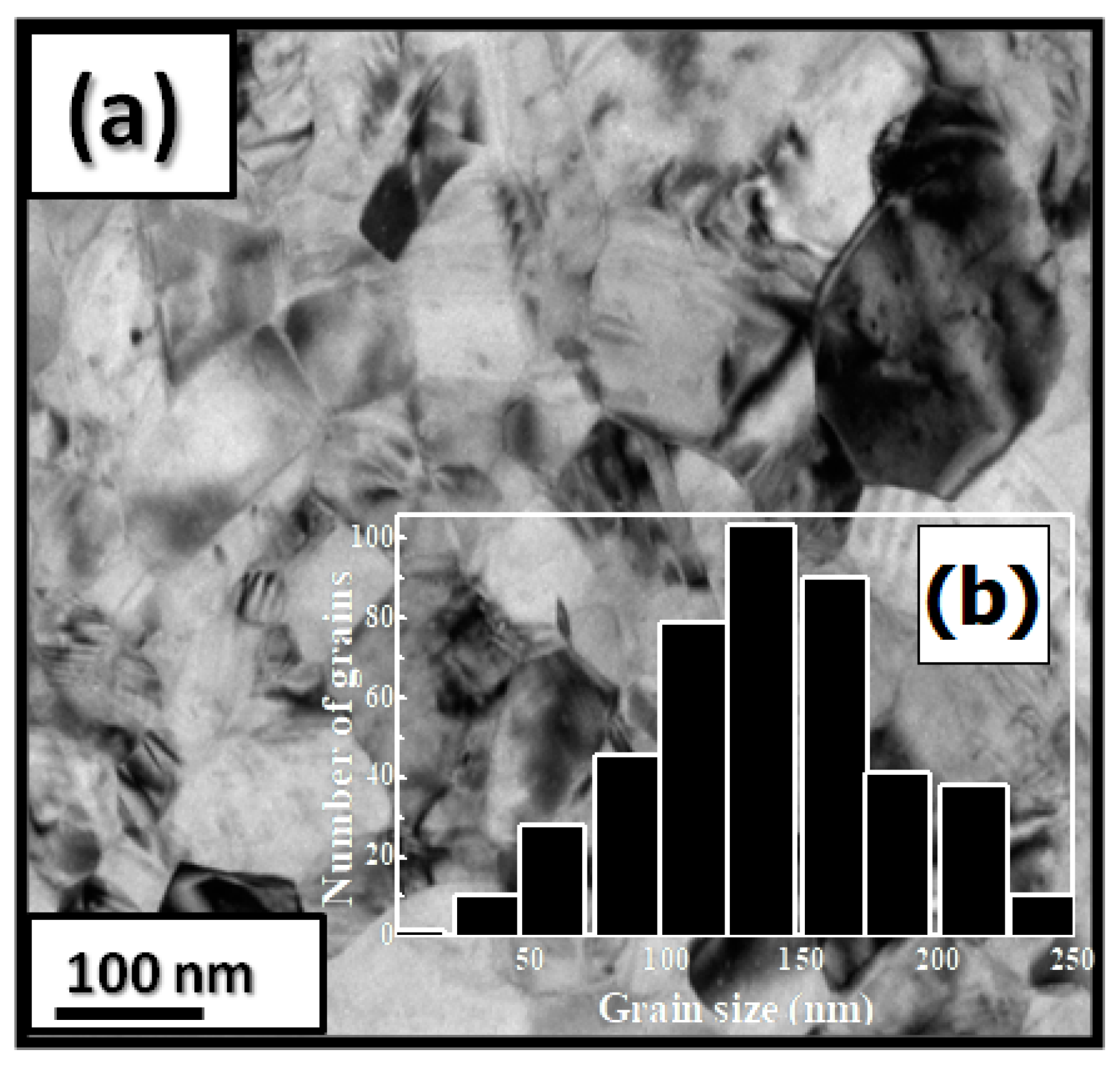
Figure 4. Bright-field micrographs of Pr 2 Co 7 C 0.75 (a). The grain size distribution is shown in the inset (b).
5. Intrinsic Magnetic Properties
5.1. Nanocrystalline Pr Co Fe (x = 0, 0.25, 0.5, 0.75, and 1) Compounds
To study the effects of Co substitution by Fe on the intrinsic magnetic properties of the alloy samples, the Curie temperature T for the nanocrystalline Pr 2 Co 7 − x Fe x phase was measured for each Fe content x. The nanocrystalline Pr 2 Co 7 − x Fe x compounds were ferromagnetic. The T C value increased from 600 K for the nanocrystalline Pr 2 Co 7 − x compound, attaining a maximum of 760 K at x = 0.5, and then decreased for x = 0.75 and x = 1. The T C values were around 695 and 667 K, respectively. The T C value was the result of two effects: the electronic effect related to the filling of the band of Fe atoms and the magnetovolume effect related to the Co-Co distances [71]. The M(H) curves of the nanocrystalline Pr 2 Co 7 − x Fe x compounds were measured at 293 K. The determination of was performed using the law of approach to saturation [77]:
The M S values were converted into the magnetic moment per formula unit .The μ s increased linearly with increasing the Fe content x. The theoretical equation of μ s as a function of x was defined by the formula: + 7x (0 ≤ x ≤ 1), where is the saturation moment of the Pr 2 Co 7 − x compound (in /f.u). The experimental data corresponded well to the theoretical forecast. The evolution of μ s with x could be justified by the reduction in the average number of 3d electrons when replacing the Co atoms with Fe, which led to a progressive strengthening of the Co(3d)-Co(3d) exchange interactions and, thus, to an enhanced magnetic moment with the 3d [71]. We realized the XRD patterns of Pr 2 Co 7 − x Fe x powders that were magnetically aligned at T = 293 K in a field of 0.5 T. For samples with x < 0.5, the (00l) reflections were heavily enhanced, indicating EMD along the c axis. For x > 0.5, the diagram shows a lower intensity of the (00l) peaks, while the reflections (h0l) are reinforced, implying that the EMD of the Pr 2 Co 7 − x Fe x sample deviates from the c axis.
5.2. Nanocrystalline Pr Co C (x = 0–1) Compounds
The Curie temperature T C of the Pr 2 Co compounds was determined according to the Co-Co, Pr-Co, and Pr-Pr interactions. In general, the Co-Co interaction was dominant, and the Pr-Co and Pr-Pr interactions were negligible. From the obtained structural data, the C insertion led to a change in the a, c parameters of the Pr 2 Co 7 compound and thus to an increase in the unit cell volume V of 2.5%. The a, c parameters varied from Å and Å for Pr 2 Co 7 to Å and Å for Pr 2 Co 7 − x C. Consequently, the Co( )-Co( 12 k ), Co( 12 k )-Co( ), and Co( 4 f )-Co( 4 f ) distances increased the related , and exchange interactions. As a result, the T C increased due to the magnetovolume effect.
5.3. Nanocrystalline Pr Co H (x = 0–10.8) Hydrides
The H absorption resulted in a variation of the lattice parameters of the Pr 2 Co 7 compound and, therefore, an increase in the cell volume V. The nearest-neighbor number of each Co atom and the Co-Co distances were then determined depending on the H content. The Co-Co distances most influenced by H absorption were those involving the Co and Co atoms, respectively, in the 4f and 12k sites. Therefore, the increase in the Co 5 -Co 5 , Co 3 -Co 5 ,Co 3 -Co 3 , distances led to a modification of the exchange interactions J ,which had some impact on the increase in T C . T C increased as a function of H content from 600 K at x = 0 to 691 K at x = 3.75, then decreased for x > 3.75. The Pr 2 Co 7 H x hydrides presented a ferromagnetic comportment for all H contents. The XRD patterns of the magnetically aligned Pr 2 Co 7 H x hydride powder samples showed only (0 0 l)-type peaks, implying that the EMDs of Pr 2 Co 7 H x hydrides were along the c axis.
5.4. Mean Field Theory (MFT) and Random Magnetic Anisotropy (RMA) Analysis
5.4.1. Nanocrystalline Pr 2 Co 7 H x (x = 0–1) Compounds
The ferromagnetic alignment of the magnetic moments of Pr and Co atoms, M and M , can be described using the following expression:
M P r and M C o were estimated using mean field theory (MFT). The magnetization M can be determined using expression (5). M C o and M P r were expected to follow the Brillouin functions [78]. M(T) was fitted for different C contents x. The experimental data align well with the theoretical curves. T C was found to be in agreement with the experimentally derived temperature. M P r , M C o and M were also determined for Pr 2 Co 7 C (Figure 9a). The J and J exchange interactions increased as a function of x, while J was found to be constant around 2.10 J. Inserting C into the Pr 2 Co 7 induced an enhancement of T C rom 600 to 730 K when x was increased from 0 to 1 (Figure 9b). The increase in T C can be ascribed to electronic and magnetovolumic effects.
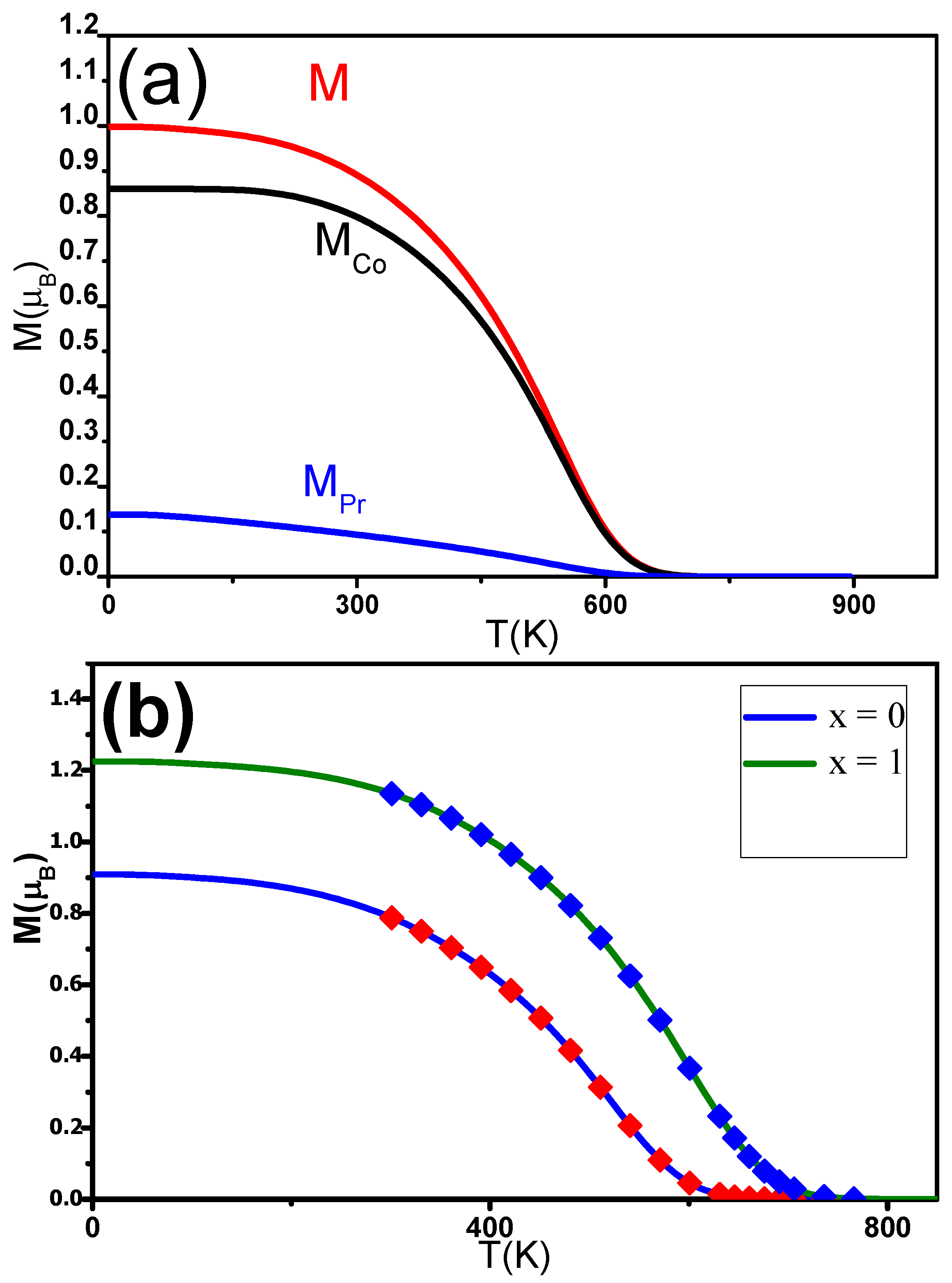
Figure 9. (a) Calculated change in the magnetic moments (M (T), M C o (T)) and magnetization (M(T)) of the nanocrystalline Pr 2 Co 7 C 0.25 compound. (b) The solid lines are the M(T) of nanocrystalline Pr 2 Co 7 H x found calculated in the MFT analysis. The symbols show the experimental data.
5.4.2. Nanocrystalline Pr 2 Co 7 H x (x = 0–10.08) Compounds
The exchange interactions of nanocrystalline Pr 2 Co 7 H x were calculated as a function of H content using the MFT . J C o − C o and J C o − P r increased when the H content increased. The exchange interaction J was around 2 × 10 J. M (T), M (T), and M(T) were fitted. The T C values were found to be in good agreement with the experimentally determined values. T C increased as a function of H content from 600 K at x = 0 to 691 K at x = 3.75, dealing with an increase of about 9.1%, then decreasing for x > 3.75. The peak value appearance of T C at x = 3.75 resulted from the H content dependence of the exchange interaction parameter of J C o − C o . The enhancement of the T C of Pr 2 Co 7 H x hydrides for x ≤ 3.75 could be the result of two effects: the electronic effect associated with a decrease in the hybridization of Co(3d) atoms with their neighbors and the magnetovolume effect associated with Co-Co distances, which suggests a decrease in antiferromagnetic interaction [57]. The Co-Co exchange interactions increased from 166 × 10 J for x = 3.75. The decrease in T C for x > 3.75 was mostly due to the reduction in Co-Co exchange interactions from 178 × 10 − 23 to 130 × 10 − 23 J for x = 10.8.−23
1−23
2
