
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | César Eduardo Falconi | -- | 7118 | 2022-04-11 18:22:09 | | | |
| 2 | Bruce Ren | -489 word(s) | 6629 | 2022-04-12 03:42:26 | | |
Video Upload Options
The lupin (Lupinus mutabilis Sweet) is a legume domesticated and cultivated for more than 4000 years by the pre-Hispanic cultures of the Andean zone. Due to its good taste and protein content, the lupin seed contributes significantly to the food and nutritional security of the Andean population. However, lupin is susceptible to diseases, and of these, anthracnose is the most devastating as it affects the whole crop, including leaves, stems, pods, and seeds. Seed treatment with fungicides reduces transmission from seed to seedling, but it does not eradicate anthracnose. Attention is given to alternative strategies from sowing to harvest to limit this seed borne pathogen as well as to enhance plant resistance and to promote plant growth. For anthracnose management in the field, integrated practices that encompass control of volunteer plants, lupin ontogenetic resistance, and rotation of biocontrol with chemical fungicides at susceptible phenological stages are considered.
1. Introduction
2. Andean Lupin
2.1. Common Names
2.2. Origin
2.3. Seed Yield Production
2.4. Nutritive Value and Potential Pharmacological Use
| L. mutabilis * | Glycine max | |
|---|---|---|
| Crude protein (% of DM) | 44.3 | 33.4 |
| Oil (%) | 16.5 | 16.4 |
| Non-starch polysaccharides (%) | 28.2 | 18.0 |
| Crude fiber (% of DM) | 7.5 | 6.45 |
| Essential amino acids (g/16 gN) | ||
| Glutamic acid | 13.79 | 15.5 |
| Aspartic acid | 6.85 | 11.0 |
2.5. Andean Lupin in Livestock Farming Systems
3. Methods to Eradicate the Pathogen from Infected Seed as the Primary Means of Pathogen Survival
3.1. Traditional Methods for Seed Disinfection
3.2. Alternative Methods for Seed Disinfection
3.2.1. Dry Heat
| Method | Doses—Exposure Time | Infection reDuction Efficiency | Additional Benefit on Lupin Plant |
|---|---|---|---|
| Dry heat | 65 °C—8 h | 75% | Promote seedling emergence [33] |
| UV-C | 57.6 kJ/m2 | 90% | Enhance physiological and biochemical processes in seedlings [34] |
| UV-B + thermal radiation |
2.83 kJ/m2 around 76 °C—45 min |
95% | Seed invigoration, positive response in plant developed, increase activity of antioxidant enzymes [35] |
| B. subtilis | Living cells (LC) from 72-h-old cultures, 109 CFU/mL or cell-free compounds (CFC) | 76 to 100% (LC), 68 to 96 (CFC) |
Positive effect on plant physiology, stimulate host defense and growth mechanisms [36][37][38] |
2.2.2. UV-C Radiation
2.2.3. UV-B + Thermal Radiation
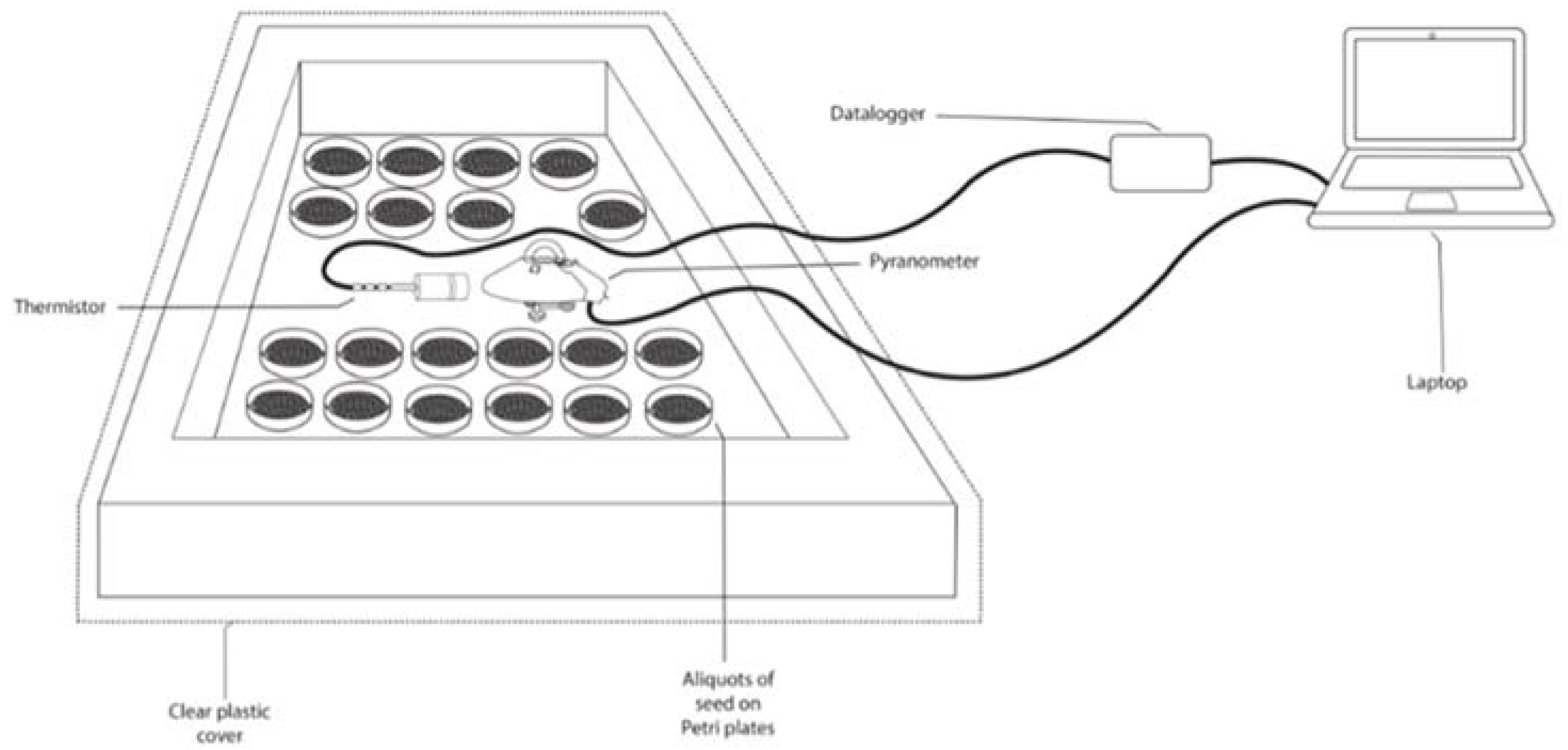
2.2.4. Biological Treatment for the Seeds
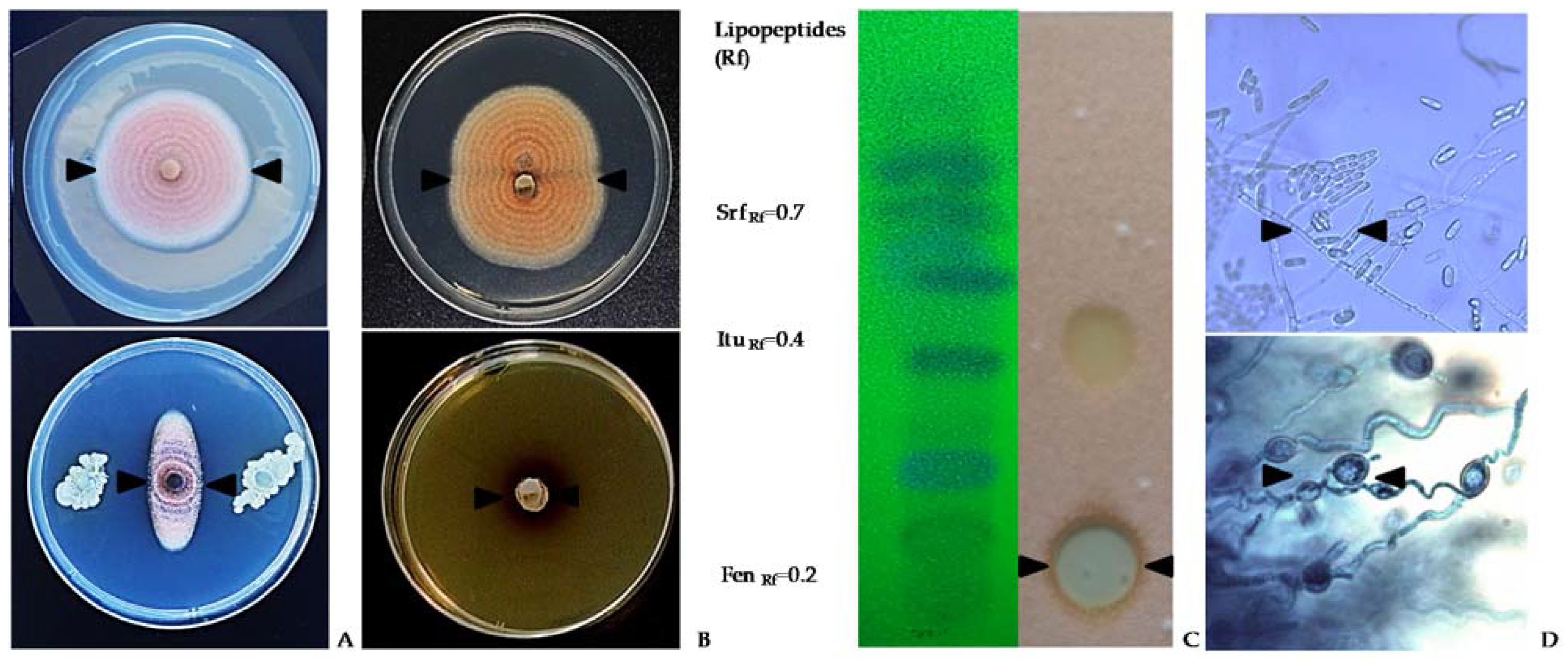
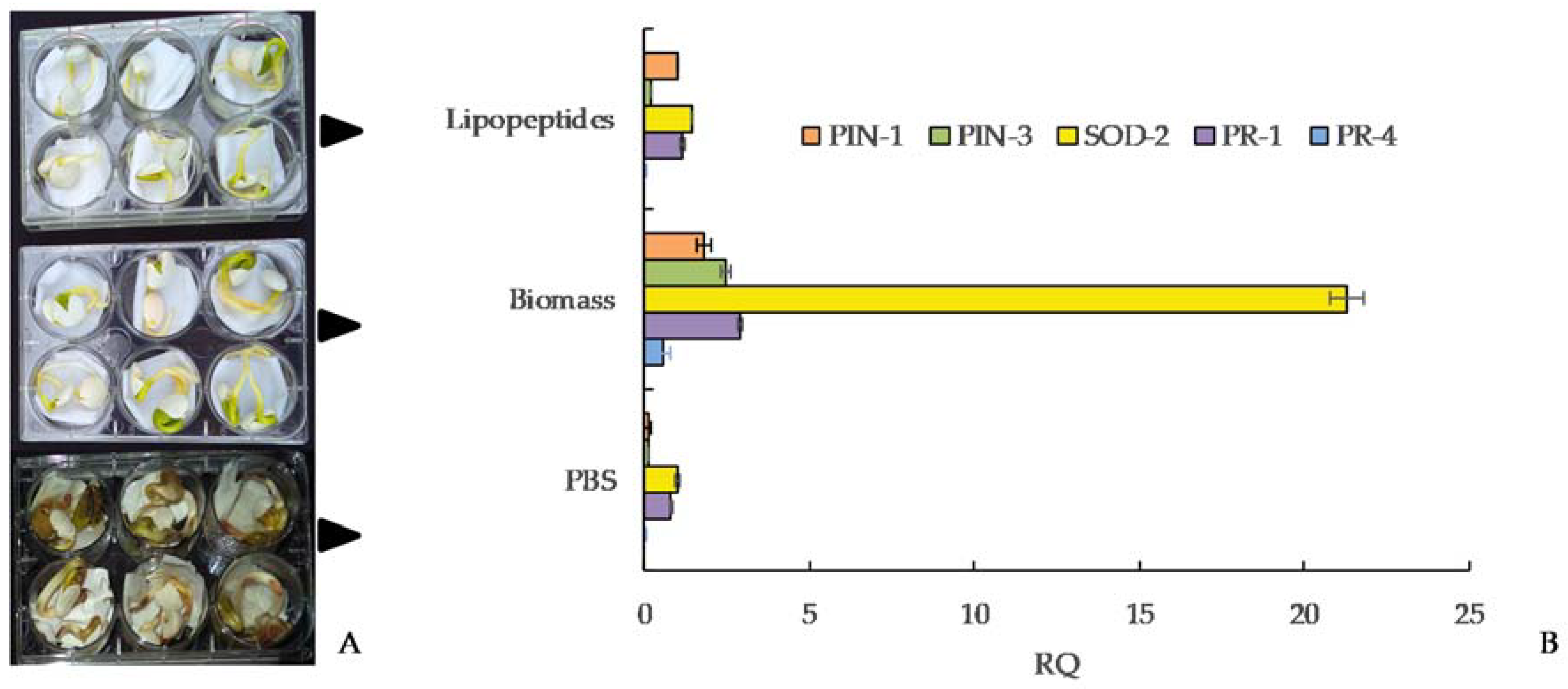
4. Strategies to Suppress Secondary Infection and Pathogen Spread
4.1. Biological Control in the Field
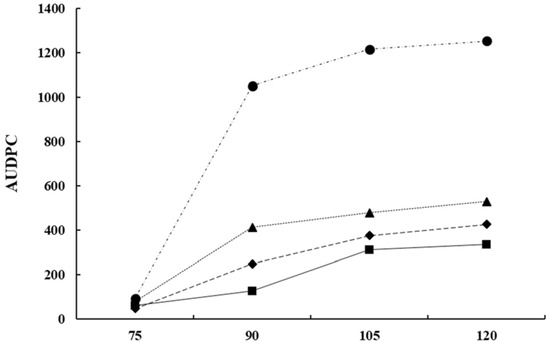
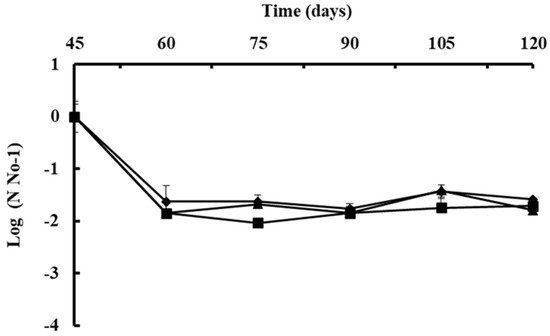
4.2. Chemical Control
| ctive Ingredient | Chemical or Biological Group | Target Site * | FRAC Code ** | References |
|---|---|---|---|---|
| Iprodione | dicarboximides | MAP/Histidine-Kinase in osmotic signal transduction | E3/2 | [99][100] |
| Procymidone | dicarboximides | MAP/Histidine-Kinase in osmotic signal transduction | E3/2 | [99][100] |
| Carbendazim | benzimidazoles | ß-tubulin assembly | B1/1 | [99][100] |
| Difeconazole | triazoles | C14- demethylase in sterol biosynthesis | G1/3 | [99] |
| Copper | inorganic | Multi-site contact activity | M 01 | [99] |
| Mancozeb, Maneb | dithiocarbamates | Multi-site contact activity | M 03 | [99][100][101] |
| Captan | phthalimides | Multi-site contact activity | M 04 | [99] |
| Folpet | chloronitriles | Multi-site contact activity | M 05 | [99] |
| Clorotalonil | chloronitriles | Multi-site contact activity | M 05 | [99][101] |
| Dithianon | quinones | Multi-site contact activity | M 05 | [103][104] |
| Ciprodinil + Fludioxonil |
anilino-pyrimidines + phenylpyrroles | Multi-site contact activity | M 09 | [104] |
| Fenhexamid + Tebuconazole |
hydroxyanilides+ triazoles | 3-keto reductase, C4- de-methylation + C14- demethylase in sterol biosynthesis |
G3/17 + G1/3 |
[104] |
| Azoxistrobin | QoI-fungicides | Complex III: cytochrome bc1 |
C3/11 | [99][100][101] |
| Pyraclostrobin + Boscalid |
methoxy-carbamates + pyridine-carboxamides | Complex III: cytochrome bc1 at Qo site + complex II: succinate-dehydrogenase | C3/11 + C2/7 |
[102] |
| Azoxystrobin + Difeconazole | QoI-fungicides + triazoles |
Complex III: cytochrome bc1 at Qo site + C14- demethylase in sterol biosynthesis | C3/11 + G1/3 |
[102] |
| B. subtilis | microbial | Multiple effects antibiosis, membrane disruption byfungicidal lipopeptides, lytic enzymes, induced plant defense | BM 02 | [38][84][106] |
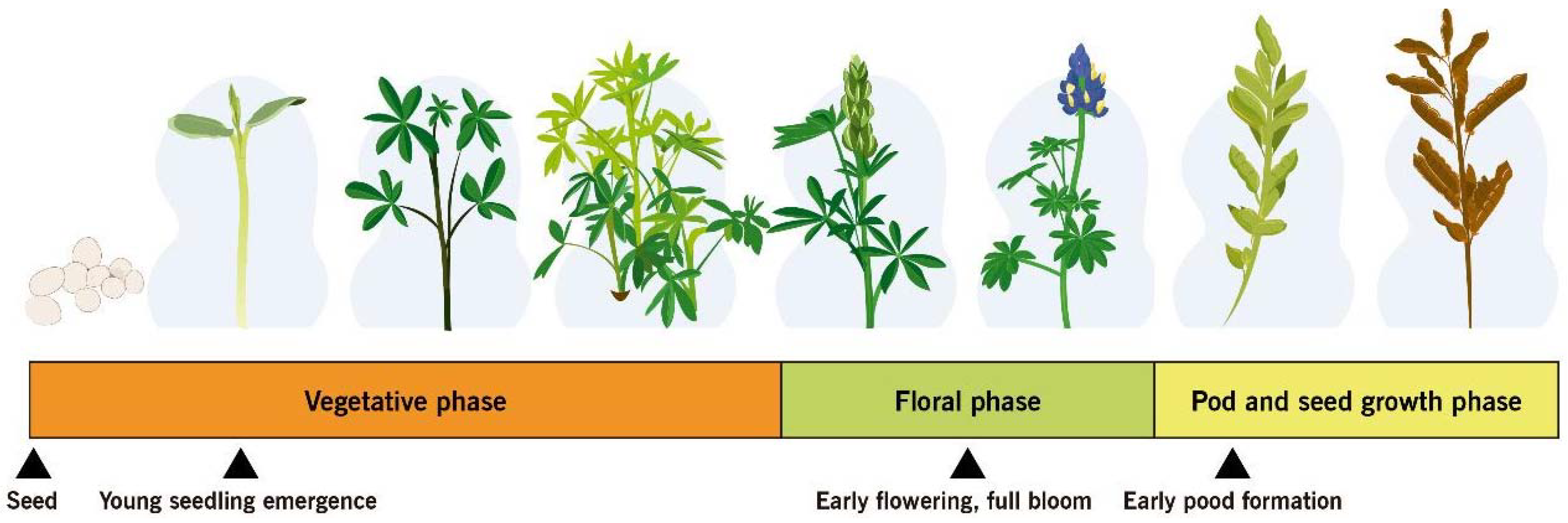
4.3. Control Volunteer Lupins and Alternative Hosts
4.4. Ontogenic Resistance
4.5. Integrated Methods at Critical Phenological Stages
References
- Mujica, A.; Moscoso, G. La planta del tarwi. In Lupinus mutabilis (Tarwi) Leguminosa Andina Con Gran Potencial Industrial; Zavaleta, A.I., Ed.; Fondo Editorial de la Universidad Nacional Mayor de San Marcos: Lima, Perú, 2018; p. 11. 164p.
- Tapia, M.E. El Tarwi, Lupino Andino. Tarwi, Tauri, o Chocho (Lupinus mutabilis Sweet); Corporación Gráfica Universal SAC: Huaylas, Perú, 2015; p. 115.
- Peralta, E.; Mazón, N.; Murillo, A.; Rivera, M.; Monar, C. Manual Agrícola de Granos Andinos. Chocho, Quinua, Amaranto y Ataco. Cultivos, Variedades, Costos de Producción; Manual No. 69; INIAP: Quito, Ecuador, 2008; 71p.
- Caicedo, C.; Peralta, E.; Villacrés, E.; Rivera, M. Postcosecha y Mercadeo de Chocho (Lupinus mutabilis Sweet) en Ecuador; Publicación Miscelánea No. 105; INIAP: Quito, Ecuador, 2001; 50p.
- Vegas-Niño, R.; Vegas-Pérez, C.; Peña-Suasnabar, C. Potencial tecnológico de la semilla de tarwi. In Lupinus mutabilis (Tarwi) Leguminosa Andina Con Gran Potencial Industrial; Zavaleta, A.I., Ed.; ondo Editorial de la Universidad Nacional Mayor de San Marcos: Lima, Perú, 2018; p. 61.
- Jacobsen, S.; Mujica, A. El tarwi (Lupinus Mutabilis Sweet.) y sus Parientes Silvestres. Botánica Económica de los Andes Centrales; Moraes, R., Øllgaard, B., Kvist, L.P., Borchsenius, F., Balslev, H., Eds.; Universidad Mayor de San Andrés: La Paz, Bolivia, 2006; pp. 458–482.
- León, J. Plantas Alimenticias Andinas; Boletín Técnico # 6; Instituto Interamericano de Ciencias Agrícolas: Zona Andina, Lima, Perú, 1964.
- Carrillo, E. Revisión del Género Lupinus en el Perú. Ph.D. Thesis, Universidad Nacional de San Agustín, Arequipa, Perú, 1956.
- Antúnez de Mayolo, S. Tarwi in Ancient Perú. In Proceedings of the First International Lupine Workshop, Lima-Cusco, Perú, 12–21 April 1982.
- Vicente, J. El cultivo de Tarwi (Lupinus mutabilis Sweet) en el Estado Plurinacional de Bolivia. Rev. Científica Investig. INFO-INIAF 2016, 1, 88–100.
- Mazón, N. El Chocho o tarwi Como Recurso Genético de la Región Andina ; Interaprendizaje—IPDRS: Quito, Ecuador, 29 October 2018; Available online: https://bit.ly/2rsbfFF (accessed on 20 January 2022).
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Int. J. Mol. Sci. 2019, 20, 851.
- French, R.J. Chapter Lupin: Agronomy. Ref. Modul. Food Sci. 2016, 1–9.
- FAOSTAT Data, Lupin Yield, Australia and Western Europe, Crops and livestock products. Food and Agriculture Organization of the United Nations, 2019. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 February 2022).
- MINAGRI—Ministerio de Agricultura y Riego. Anuario Estadístico de la Producción Agrícola y Pecuaria 2017; III Censo Nacional Agropecuario; MINAGRI: Quito, Ecuador, 2018.
- Tello, F. Lupinus mutabilis sweet-a potent food source from the Andean region. Am. J. Clin. Nutr. 1976, 29, 933.
- Wallace, C.; Buirchell, B.J.; Tapia, M.E.L.; Lupinus, L. Promoting the Conservation and Use of Underutilized and Neglected Crops. 23; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1998; 104p.
- Von Baer, E. Protein and oil content and yield of L. mutabilis in South America and Westhern Europe. In Proceedings of the 6th International Lupin Conference, Chilean Lupin Association, Temuco, Chile, 25–30 November 1991; pp. 13–15.
- Gross, R. El Cultivo y la Utilización del tarwi (Lupinus mutabilis Sweet); No. 581.632 F3; FAO: Rome, Italy, 1981.
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705.
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; et al. State and Progress of Andean Lupin Cultivation in Europe: A Review. Agronomy 2020, 10, 1038.
- Petterson, D.S.; Sipsas, S.; Mackintosh, J.B. The Chemical Composition and Nutritive Value of Australian Grain Legumes, 2nd ed.; Grain Research and Development Corporation: Camberra, Australia, 1997.
- Camarena, F. El Cultivo del Tarwi; Programa de leguminosas de Grano; Universidad Nacional Agraria La Molina: Lima, Perú, 2000.
- Villacrés, E.; Cuadrado, L.; Falconí, F. Los Granos Andinos: Chocho (Lupinus mutabilis Sweet), Quinua (Chenopodium quinua Willd), Amaranto (Amaranthus caudatus L.) y Sangorache (Amaranthus hybridus L.), fuente de Metabolitos Secundarios y Fibra Dietética; Boletín Técnico No. 165; INIAP- Estación Experimetal Santa Catalina: Quito, Ecuador, 2013; 42p.
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus spp. Sustainability 2018, 10, 788.
- Villacrés, E.; Cueva, P.; Díaz, M.; Rosell, C.M. Replacing Wheat Flour with Debittered and Fermented Lupin: Effects on Bread’s Physical and Nutritional Features. Plant Foods Hum. Nutr. 2020, 75, 569–575.
- Fornasini-Salvador, M.V.; Abril-Ulloa, S.V.; Beltrán-Carreño, J.P.; Elena Villacrés, E.; Cuadrado-Merino, L.; Robalino, F.; Sánchez, R.; Ricaurte-Ortiz, P.S.; Muñoz, E.B.; Benítez-Loza, N.B.; et al. Efficacy of a Lupinus mutabilis Sweet snack as complement to conventional type 2 diabetes mellitus treatment. Nutr. Hosp. 2019, 36, 905–911.
- Canahua, A.; Roman, P. Tarwi. Leguminosa andina de gran potencial. Rev. De Agroecol. LEISA 2016, 32, 2. Available online: https://www.servindi.org/actualidad-opinion/27/07/2016/tarwi-leguminosa-andina-de-gran-potencial (accessed on 13 January 2022).
- Falconi, C.E. Lupinus mutabilis in Ecuador with Special Emphasis on Anthracnose Resistance. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2012; 150p. Available online: https://edepot.wur.nl/210228 (accessed on 18 February 2022).
- Thomas, G.; Jones, R.; Vanstone, V. Diseases of lupin. In Producing Lupins, 2nd ed.; White, P., French, B., McLarty, A., Eds.; Department of Agriculture and Food: Bulletin, WA, USA, 2008; pp. 101–120. ISSN 1833-7236.
- Thomas, G.J.; Sweetingham, M.W. Fungicide seed treatments reduce seed transmission and severity of lupin anthracnose caused by Colletotrichum gloeosporioides. Australas. Plant Pathol. 2003, 32, 39–46.
- Thomas, G.J.; Adcock, K.G. Exposure to dry heat reduces anthracnose infection of lupin seed. Australas. Plant Path 2004, 33, 537–540.
- Falconí, C.E.; Yánez-Mendizábal, V. Dry heat treatment of Andean lupin seed to reduce anthracnose infection. Crop Prot. 2016, 89, 178–183.
- Falconí, C.E.; Yánez-Mendizábal, V. Efficacy of UV-C radiation to reduce seedborne anthracnose (Colletotrichum acutatum) from Andean lupin (Lupinus mutabilis). Plant Pathol. 2017, 67, 831–838.
- Falconí, C.E.; Yánez-Mendizábal, V. Solar UV-B radiation limits anthracnose infection in seed and promote biochemical and physiological response in lupin plants. Plant Pathol. 2019, 68, 1635–1644.
- Yánez-Mendizábal, V.; Falconí, C.E.; Grijalva, A.C. Bacillus spp. evaluation to control anthracnose infection on Andean lupin seed (Lupinus mutabilis Sweet). Phytopathology 2015, 105, S4–S153.
- Yánez-Mendizábal, V.; Falconí, C.E. Efficacy of spp. to biocontrol of anthracnose and enhance plant growth on Andean lupin seeds by lipopeptide production. Biol. Control 2018, 122, 67–75.
- Yánez-Mendizábal, V.; Falconí, C.E. Bacillus subtilis CtpxS2-1 induces systemic resistance against anthracnose in Andean lupin by lipopeptide production. Biotechnol. Lett. 2021, 43, 719–728.
- Cowling, W.A. Pedigrees and Characters of Narrow-Leafed Lupin Cultivars Released in Australia from 1967 to 1998; Lupin Breeding Bulletin 4365; Agriculture Western Australia: Perth, Australia, 1999.
- Sweetingham, M.; Clements, J.; Buirchell, B.; Sipsas, S.; Thomas, G.; Quealy, J.; Jones, R.; Francis, C.; Smith, C.G. Preliminary breeding and development of Andean lupin for Australian agriculture. In Where Old and New World Lupins Meet, Proceedings of the 11th International Lupin Conference, Guadalajara, Jalisco, Mexico, 4–9 May 2005; Van Santen, E., Hill, G.D., Eds.; International Lupin Association: Canterbury, New Zealand, 2005.
- Gulisano, A.; Alves, S.; Neves Martins, J.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1–13.
- Basra, S.M.A.; Ashraf, M.; Iqbal, N.; Khaliq, A.; Ahmed, R. Physiological and biochemical aspects of pre-sowing heat stress on cotton seed. Seed Sci. Technol. 2004, 32, 765–774.
- Chevrefils, G.; Caron, E.; Wright, H.; Sakamoto, G.; Payment, P.; Barbeau, B.; Cairns, B. UV dose required to achieve incremental log inactivation of bacteria, protozoa and viruses. IUVA News 2006, 8, 38–45.
- Bokhari, N.A.; Siddiqui, I.; Parveen, K.; Siddique, I.; Rizwana, H.; Soliman, D.A. Management of anthracnose of banana by UV radiation. J. Anim. Plant Sci. 2013, 23, 1211–1214.
- González-Aguilar, G.A.; Wang, C.Y.; Buta, J.G.; Krizek, D.T. Use of UV- C radiation to prevent decay and maintain postharvest quality of ripe ‘Tommy Atkins’ mangoes. Int. J. Food Sci. Technol. 2001, 36, 767–773.
- Wilson, C.L.; Heidinger, G.F. Ultraviolet Light Treatments for Increasing Seed Yields. U.S. Patent WO2010085513 A1, 29 July 2010.
- Darras, A.I.; Demopoulos, V.; Tiniakou, C. UV-C radiation induces defense responses and improves vase-life of cut gerbera flowers. Postharvest Biol. Technol. 2012, 64, 168–174.
- Shetta, N.D.; Areaf, I.M. Impact of ultraviolet-C radiation on seed germination and chlorophyll concentration of some woody trees grown in Saudi Arabia. J. Agric. Env. Sci 2009, 8, 1–20.
- Middleton Middleton, E.M.; Teramura, A.H. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993, 103, 741–752.
- Peykarestan, B.; Seify, M. UV irradiation effects on seed germination and growth, protein content, peroxidase and protease activity in redbean. Int. Res. J. Appl. Basic. Sci. 2012, 3, 92–102.
- Hieng, B.; Ugrinovic, K.; Sustar-vozlic, J.; Kidric, M. Different classes of proteases are involved in the response to drought of Phaseolus vulgaris L. cultivars differing in sensitivity. J. Plant Physiol. 2004, 161, 519–530.
- Salama, H.M.H.; Watban, S.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 12, 1–26.
- Rivera-Pastrana, D.M.; Gardea, A.A.; Yahia, E.M.; Martínez-Téllez, M.A.; González-Aguilar, G.A. Effect of UV-C irradiation and low temperature storage on bioactive compounds, antioxidant enzymes and radical scavenging activity of papa fruit. J. Food Sci. Technol. 2014, 51, 3821–3829.
- Glazenner, J.A. Accumulation of phenolic compounds in cells and formation of lignin-like polymers in cell walls of young tomato fruits after inoculation with Botrytis cinerea. Physiol. Plant Pathol. 1982, 20, 11–25.
- Anand, T.A.; Chandrasekaran, A.; Kuttalam, S.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J. Plant Interact. 2007, 2, 233–244.
- Kanetis, L.; Holmes, G.J.; Ojiambo, P.S. Survival of Pseudoperonospora cubensis sporangia exposed to solar radiation. Plant Pathol. 2010, 59, 313–323.
- Rotem, J.; Wooding, B.; Aylor, D.E. The role of solar radiation, especially ultraviolet, in the mortality of fungal spores. Phytopathology 1985, 75, 510–514.
- Mizubuti, E.S.G.; Aylor, D.E.; Fry, W.E. Survival of Phytophthora infestans sporangia exposed to solar radiation. Phytopathology 2000, 90, 78–84.
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Biol. 1991, 36, 299–328.
- De Menezes, H.D.; Massola, N.S., Jr.; Flint, S.D.; Silva, G.J., Jr.; Bachmann, L.; Rangel, D.E.N.; Braga, G.U.L. Growth under visible light increases conidia and mucilage production and tolerance to UV-B radiation in the plant pathogenic fungus Colletotrichum acutatum. Photochem. Photobiol. 2015, 91, 397–402.
- Begum, M.; Sariah, M.; Puteh, A.; Zainal, A. Detection of seed-borne fungi and site of infection by Colletotrichum truncatum in naturally-infected soybean seeds. Int. J. Agric. Res. 2007, 2, 812–819. Available online: https://scialert.net/abstract/?doi=ijar.2007.812.819 (accessed on 15 January 2022).
- Tepfer, D.; Leach, S. Survival and DNA damage in plant seeds exposed for 558 and 682 days outside the International Space Station. Astrobiology 2017, 17, 205–215.
- Noble, R.E. Effects of UV-irradiance on seed germination. Sci. Total Environ. 2002, 299, 173–176.
- Shaukat, S.; Farooq, M.; Siddiqui, M.; Zaidi, S. Effect of enhanced UV-B radiation on germination, seedling growth and biochemical responses of Vigna mungo (L.) Hepper. Pak. J. Bot 2013, 45, 779–785.
- Araújo, S.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646.
- Sugimoto, K. Seed germination under UV-B irradiation. Bull. Minamikyushu Univ. 2013, 43, 1–9.
- Hideg, É.; Hideg, E.; Rosenqvist, E.; Váradi, G.; Bornman, J.F.; Vincze, E. A comparison of UV-B induced stress responses in three barley cultivars. Funct. Plant Biol. 2006, 33, 77–90.
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071.
- Caldwell, M.M.; Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266.
- Ravindran, K.C.; Indrajith, A.; Pratheesh, P.V.; Sanjiviraja, K.; Balakrishnan, V. Effect of ultraviolet-B radiation on biochemical and antioxidant defense system in Indigofera tinctoria L. seedlings. Int. J. Eng. Sci. Technol. 2012, 2, 226–232.
- Yánez-Mendizábal, V.; Zeriouh, H.; Viñas, I.; Torres, R.; Usall, J.; De Vicente, A.; Pérez-García, A.; Teixido, N. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur. J. Plant Pathol. 2012, 132, 609–619.
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125.
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis towards Podosphaera fusca. Mol. Plant Microbe Interact. 2007, 20, 430–440.
- Ongena, M.; Henry, G.; Thonart, P. The roles of cyclic lipopeptides in the biocontrol activity of Bacillus subtilis. In Recent Developments in Management of Plant Diseases, Plant Pathology in the 21st Century, Proceedings of the 9th International Congress of Plant Pathology, Torino, Italy, 24–29 August 2008; Gisi, U., Chet, L., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2009.
- Miles, T.; Nair, M.; Vandervoort, C.; Schilder, A.M.C. Characterization and biological activity of flavonoids from ripe fruit of an anthracnose resistant blueberry cultivar. Physiol. Mol. Plant Pathol. 2013, 83, 8–16.
- Meazza, G.; Dayan, F.; Wedge, D.E. Activity of Quinones on Colletotrichum Species. J. Agric. Food Chem. 2003, 51, 3824–3828.
- Deleu, M.; Paquot, M.; Nylander, T. Fengycin interaction with lipid monolayers at the air–aqueous interface—implications for the effect of fengycin on biological membranes. J. Colloid Interface Sci. 2005, 283, 358–365.
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin—A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901.
- Yasmeen, S.; Sariah, M. Effect of Seed Bacterization on Plant Growth Response and Induction of Disease Resistance in Chilli. Agric. Sci. China 2009, 8, 963–971.
- Manjula, K.; Podile, A.R. Production of fungal cell wall degrading enzymes by a biocontrol strain of Bacillus subtilis AF1. Indian J. Exp. Biol. 2005, 43, 892–896.
- Singh, S.K.; Pathak, R.; Choudhary, V. Plant Growth-Promoting Rhizobacteria-Mediated Acquired Systemic Resistance in Plants against Pest and Diseases. In Microbial-Mediated Induced Systemic Resistance in Plants; Choudhary, D.K., Varma, A., Eds.; Springer: Singapore, 2016; pp. 125–134.
- Park, J.W.; Balaraju, K.; Kim, J.W.; Lee, S.W.; Park, K. Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol. Control 2013, 65, 246–257.
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganism 2020, 8, 1037.
- Chowdappa, P.; Kumar, S.M.; Lakshmi, M.J.; Mohan, S.P.; Upreti, K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117.
- Jayapala, N.; Mallikarjunaiah, N.; Puttaswamy, H.; Gavirangappa, H.; Ramachandrappa, N.S. Rhizobacteria Bacillus spp. induce resistance against anthracnose disease in chili (Capsicum annuum L.) through activating host defense response. Egypt. J. Biol. Pest Control 2019, 29, 45.
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684.
- Falconí, C.E.; Yánez-Mendizábal, V.; Claudio, D. Native Bacillus subtilis strains efficiently control lupin anthracnose both under greenhouse and in field conditions. Ecol. Environ. Conserv. 2022; accepted.
- Lindbeck, K.; Fanning, J.; Jason Brand, J. Diseases of Lupins. Updated on 22 June 2020. Available online: https://extensionaus.com.au/FieldCropDiseasesVic/docs/identification-management-of-field-crop-diseases-in-victoria/lupins/summary/ (accessed on 7 February 2022).
- Wang, X.Q.; Zhao, D.L.; Shen, L.L.; Jing, C.L.; Hang, C.S. Application and Mechanisms of Bacillus subtilis in Biological Control of Plant Disease. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 225–250.
- Hojin, R.; Hoon, P.; Dong-Sang, S.; Gun, H.J.; Kyungseok, P.; Byung, D.L. Biological control of Colletotrichum panacicola on Panax ginseng by Bacillus subtilis HK-CSM-1. Ginseng Res. 2014, 38, 215–219.
- Falconi, C.E.; Visser, R.G.F.; van Heusden, A.W. Influence of Plant Stage on Resistance to Anthracnose in Andean Lupin (Lupinus mutabilis Sweet). Crop Pasture Sci. 2015, 66, 729–734.
- Wei, F.; Hu, X.; Xu, X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci. Rep. 2016, 6, 22611.
- Demoz, B.T.; Korsten, L. Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stemend rot pathogens. BioControl 2006, 37, 68–74.
- Yadav, R.K.; Kakamanoli, K.; Vokou, D. Estimating bacterial population on the phyllosphere by serial dilution plating and leaf imprint method. Biol. Ecoprint: Int. J. Ecol. 2010, 17, 47–52.
- Falconi, C.E. Control Biológico de Enfermedades de las Plantas en Ecuador. Capítulo 9. In Control Biológico de Enfermedades en los Cultivos en Latinoamérica y el Caribe; Belliot, W., Mondino, P., Comenarez, Y., Vero, S., Montealegre, J., Rivera, M., Eds.; Editorial Universidad de la República: Montevideo, Uruguay, 2014; 402p, pp. 214–242.
- Falconí, C.E.; Peralvo, D.; Saavedra, L.; Yánez-Mendizábal, V. Validación de biopreparados en base a bacterias epifitas para el control de la moniliasis en cacao fino de aroma en el cantón Valencia de la provincia de Los Ríos. In Validación de Biopesticidas para el Control de la Moniliasis y Manejo Sustentable del Cacao Fino de Aroma en el Ecuador; Falconí, C., Yánez, V., Eds.; Artes Gráficas Señal: Quito, Ecuador, 2007; pp. 37–49.
- Cruz-Martín, M.; Acosta-Suárez, M.; Mena, E.; Roque, B.; Pichardo, T.; Alvarado-Capó, Y. Antifungal activity of Musa phyllosphere Bacillus pumilus strain against Mycosphaerella fijiensis. Trop. Plant Pathol. 2017, 42, 121–125.
- Thomas, G.J.; Sweetingham, M.W.; Adcock, K.G. Application of fungicides to reduce yield loss in anthracnose-infected lupins. Crop Prot. 2008, 27, 1071–1077.
- Cambo, E.I. Evaluación de Cuatro Fungicidas Sobre la Antracnosis (Colletotrichum acutatum) en el Cultivo de Chocho (Lupinus mutabilis) variedad INIAP—450. Bachelor’s Thesis, Universidad de las Fuerzas Armadas ESPE, Sangolqui, Ecuador, 2016; p. 54.
- Jílková, B.; Víchová, J.; Pokorný, R.; Vejražka, K. Sensitivity of Colletotrichum acutatum Isolates to Selected Fungicides. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 1111–1119.
- Vega, L.E.; Rodríguez, D.G.; Murillo, A.R.; Mazón, N.G. Cuantificación del Daño y Validación de Una Estrategia de Control Químico de la Antracnosis (Colletotrichum acutatum) en el Cultivo del Chocho (Lupinus mutabilis Sweet) en Ecuador; 1er Congreso Internacional Ciencia y Tecnología Agropecuaria; INIAP—Estación Experimental Santa Catalina: Quito, Ecuador, 2018; pp. 80–82.
- Shea, G.; Thomas, G.; Buirchell, B.; Salam, M.; McKirdy, S.; Sweetingham, M. Case study: Industry response to the lupin anthracnose incursion in Western Australia. In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Australia, 14–18 September 2008; Palta, J.A., Berger, J.B., Eds.; International Lupin Association: Canterbury, New Zealand, 2008; pp. 425–431.
- Silva-Junior, G.J.; Spósito, M.S.; Marin, D.R.; Amorim, L. Efficacy and timing of application of fungicides for control of citrus postbloom fruit drop. Crop Prot. 2014, 59, 51–56.
- FRAC Code List ©*2021: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2021--final.pdf?sfvrsn=f7ec499a_2 (accessed on 22 January 2022).
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815.
- Saxena, A.; Raghuwanshi, R.; Gupta, V.K.; Singh, H.B. Chilli Anthracnose: The Epidemiology and Management. Front. Microbiol. 2016, 7, 1–18.
- Förster, H.; Driever, G.F.; Thompson, D.C.; Adaskaveg, J.E. Postharvest decay management for stone fruit crops in California using the “reduced-risk” fungicides fludioxonil and fenhexamid. Plant Dis. 2007, 91, 209–215.
- Falconi, C.E.; Visser, R.G.F.; van Heusden, A.W. Phenotypic, molecular, and pathological characterization of Colletotrichum acutatum associated with lupine and tamarillo in the Ecuadorian Andes. Plant Dis. 2013, 97, 819–827.
- Wirtz, L.; Massola-Júnior, N.S.; Linhares de Castro, R.R.; Ruge-Wehling, B.; Schaffrath, U.; Loehrer, M. Colletotrichum spp. from Soybean Cause Disease on Lupin and Can Induce Plant Growth-Promoting Effects. Microorganisms 2021, 9, 1130.
- Ruales, C. Las Colecciones Botánicas de Joseph de Jussieu (1736–1747); Serie Monográfica “Plantas de Quito—La vegetación original de una ciudad siempre verde”; Universidad San Francisco de Quito: Quito, Ecuador, 2013; Volume 1, p. 293.
- Falconi, C.E.; van Heusden, A.W. Morphological, pathological and molecular characterization of lupin anthracnose and its relationship with tamarillo anthracnose in Ecuadorian Andes. American Phytopathological Society Annual Meeting, Honolulu—Hawaii. Phytopathology 2011, 101, S9–S23. Available online: https://apsjournals.apsnet.org/doi/pdf/10.1094/PHYTO.2011.101.6.S1 (accessed on 12 January 2022).
- Ficke, A.; Gadoury, D.M.; Seem, R.C. Ontogenic resistance and plant disease management: A case study of grape powdery mildew. Phytopathology 2002, 92, 671–675.
- Beate Tschöpe, P.R. SIMONTO-Lupin: An ontogenetic simulation model for lupin species (Lupinus angustifolius, L. luteus and L. albus). J. Für Kult. 2011, 63, 1626–1632.
- Sinde-González, I.; Falconí, C.E.; Luna-Granizo, P.; Godoy-Guanín, L.; Gil-Docampo, M.; Maiguashca, J.; Nato, R. Spectral analysis of the phenological stages of Lupinus mutabilis through spectroradiometry and unmanned aerial vehicle imaging with different physical disinfection pretreatments of seeds. Geocarto Int. 2021, 1–18.
- Thomas, G.J.; Sweetingham, M.W. Cultivar and environment influence the development of lupin anthracnose caused by Colletotrichum lupini. Australas. Plant Pathol. 2004, 33, 571–577.
- Yánez-Mendizábal, V.; Usall, J.; Viñas, I.; Casals, C.; Marín, S.; Solsona, C.; Teixidó, N. Potential of a new strain of Bacillus subtilis CPA-8 to control the major postharvest diseases of fruit. Biocontrol. Sci. Technol. 2011, 21, 409–426.
- Yánez-Mendizábal, V.; Viñas, Y.; Usall, J.; Torres, R.; Solsana, C.; Teixidó, N. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biol. Control 2012, 60, 280–289.
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Teixidó, N. Endospore production allows using spray-drying. As a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735.
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Abadias, M.; Teixidó, N. Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J. Appl. Microbiol. 2012, 112, 954–965.




