The lupin (Lupinus mutabilis Sweet) is a legume domesticated and cultivated for more than 4000 years by the pre-Hispanic cultures of the Andean zone. Due to its good taste and protein content, the lupin seed contributes significantly to the food and nutritional security of the Andean population. However, lupin is susceptible to diseases, and of these, anthracnose is the most devastating as it affects the whole crop, including leaves, stems, pods, and seeds. Seed treatment with fungicides reduces transmission from seed to seedling, but it does not eradicate anthracnose. Attention is given to alternative strategies from sowing to harvest to limit this seed borne pathogen as well as to enhance plant resistance and to promote plant growth. For anthracnose management in the field, integrated practices that encompass control of volunteer plants, lupin ontogenetic resistance, and rotation of biocontrol with chemical fungicides at susceptible phenological stages are considered.
- Andean lupin
- anthracnose
- integrated management
- plant protection
- dry heat
- UV-C
- UV-B
- Bacillus subtilis
- biological control
- ontogenic resistance
- chemical control
1. Introduction
2. Andean Lupin
2.1. Common Names
2.2. Origin
2.3. Seed Yield Production
2.4. Nutritive Value and Potential Pharmacological Use
| L. mutabilis * | Glycine max | |
|---|---|---|
| Crude protein (% of DM) | 44.3 | 33.4 |
| Oil (%) | 16.5 | 16.4 |
| Non-starch polysaccharides (%) | 28.2 | 18.0 |
| Crude fiber (% of DM) | 7.5 | 6.45 |
| Essential amino acids (g/16 gN) | ||
| Glutamic acid | 13.79 | 15.5 |
| Aspartic acid | 6.85 | 11.0 |
2.5. Andean Lupin in Livestock Farming Systems
3. Methods to Eradicate the Pathogen from Infected Seed as the Primary Means of Pathogen Survival
3.1. Traditional Methods for Seed Disinfection
3.2. Alternative Methods for Seed Disinfection
3.2.1. Dry Heat
| Method | Doses—Exposure Time | Infection reDuction Efficiency | Additional Benefit on Lupin Plant |
|---|---|---|---|
| Dry heat | 65 °C—8 h | 75% | Promote seedling emergence [45][33] |
| UV-C | 57.6 kJ/m2 | 90% | Enhance physiological and biochemical processes in seedlings [51][34] |
| UV-B + thermal radiation |
2.83 kJ/m2 around 76 °C—45 min |
95% | Seed invigoration, positive response in plant developed, increase activity of antioxidant enzymes [56][35] |
| B. subtilis | Living cells (LC) from 72-h-old cultures, 109 CFU/mL or cell-free compounds (CFC) | 76 to 100% (LC), 68 to 96 (CFC) |
Positive effect on plant physiology, stimulate host defense and growth mechanisms [57,58,59][36][37][38] |
2.2.2. UV-C Radiation
2.2.3. UV-B + Thermal Radiation
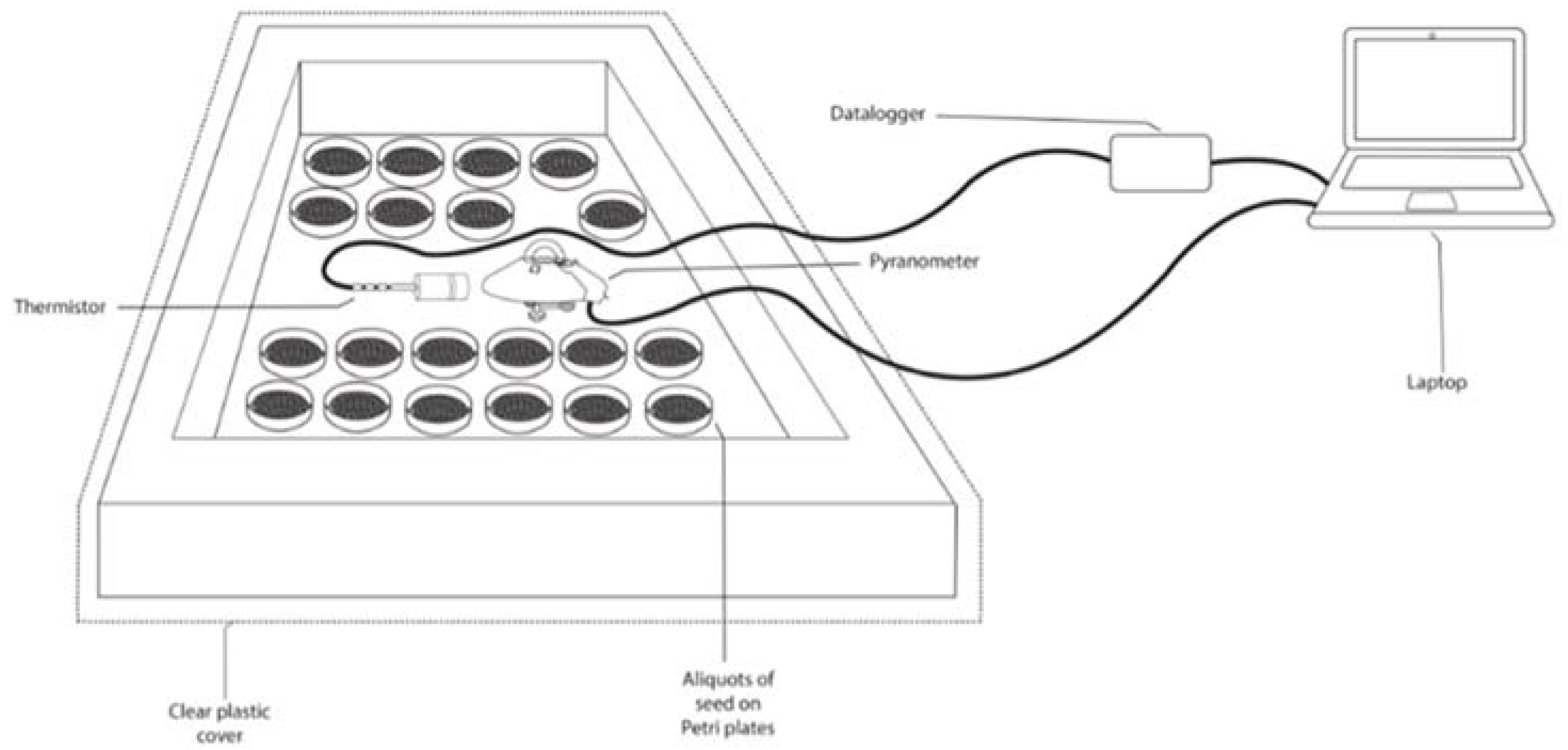
2.2.4. Biological Treatment for the Seeds
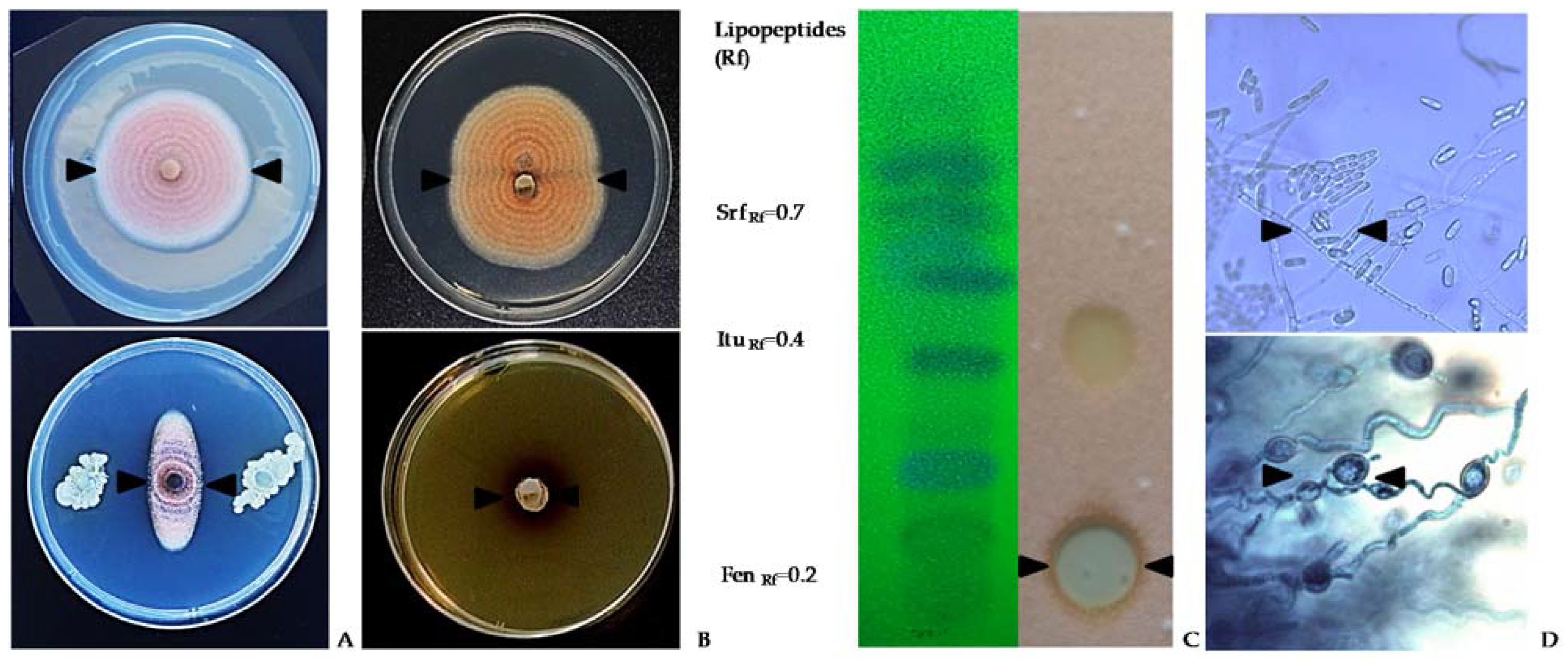
4.1. Biological control in the field
From a commercial point of view, the use of Bacillus-based preparations has been widely demonstrated efficient for controlling multiple field fungal pathogens [95]. However, research on the biocontrol of lupin anthracnose is scarce. Studies conducted in the field with anthracnose natural inoculum pressure showed that all B. subtilis treatments reduce the Area Under Disease Progress Curve (AUDPC) compared with controls that showed large stem lesions, accompanied by necrotic tissue 90 days after sowing (DAS) and abundant sporulation, and in some cases dead plant from 105 to 120 DAS [99]. The AUDPC was reduced with sequential applications of antagonists 1x 10
4. Strategies to Suppress Secondary Infection and Pathogen Spread
4.1. Biological Control in the Field
9 UFC/ml, every two-week, compared to the AUDPC of untreated control in susceptible cultivars (Fig. 4). In general, treatments with bacterial suspensions result in healthier stems, leaves, and pods, which are agronomic characteristics of lupin linked to production [99]. The significant reduction of AUDPC may be due to B. subtilis having a cascade effect on the different components of the disease triangle, that is, on the environment, the host plant, or the pathogen. Therefore, it can affect the onset and progress of the disease and also turn on resistance genes in the host [100]. B. subtilis induces disease resistance in the plant, or in turn promotes plant growth, a fact that makes it easier for plants to control pathogenic infections [90, 91, 93, 98] or suppress diseases based on systemic resistance [94, 95, 96, 98]. B. subtilis have also shown efficiency to control ginseng anthracnose, Colletotrichum panacicola, where leaf lesion diameter was not significantly affected compared with the fungicide treatment. The protection mechanism of B. subtilis was associated with a reduction of the incidence and with the initial processes of the infection cycle such as spore germination, appressorium formation, and penetration [99].
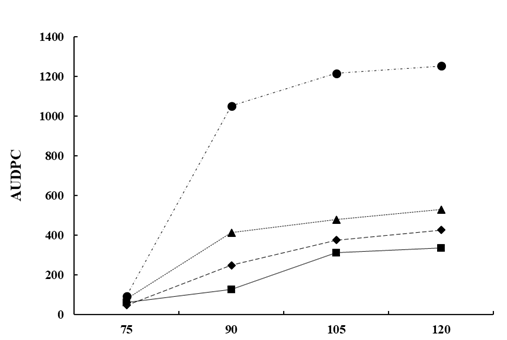
Figure 4. The Area under disease progress curve (AUDPC) of anthracnose caused by C. lupini on the Andean lupin susceptible cultivar I.450 Andino. The natural pressure of inoculum is represented as the control (Ck ) and the AUDPC by the effect of B. subtilis Ctpx-S1 ; Ctpx-S2 and Ctpx-Z3 . AUDPC calculates on scores for lupin anthracnose severity on the 1-to-6 scale [100]. Pooled data from the 2015 and 2019 growing seasons at El Chaupi, Cantón Mejía, Ecuador, each point at disease onset plus 90, 105, and 120 days. Source: [99].
A B. subtilis strain selection program generally begins with screening under controlled laboratory or semi-controlled greenhouse conditions; however, it is difficult to predict how the bacteria will respond when released into the natural environment [100]. The native bacterial population on the phylloplane was estimated to confirm its protectant effect. Viable cell concentrations of B. subtilis were reduced to about 2.0 LOG after 15 days of the first application on Andean Lupin (Fig. 5) [99] or reduced by 50% in strawberry leaves in the open field after 8 days of application [102]. Environmental stresses such as intense sunlight, dryness, and high temperature could reduce initial colonization [99, 103] or rainfall could easily remove the bacterial population from the plant surface in several hours [104]. However, the population of B. subtilis will vary to some degree naturally, once it has been established in the field. The average of native B. subtilis population on the phyllosphere of lupin remains stable at around 7.0 LOG after successive two-week-sprays for two months, which suggests that after the population has become stable, it can resist harsh environmental conditions (Fig. 5) [99].
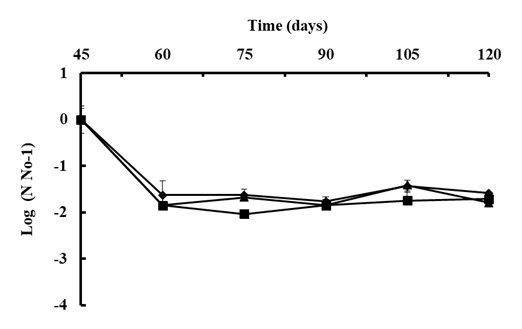
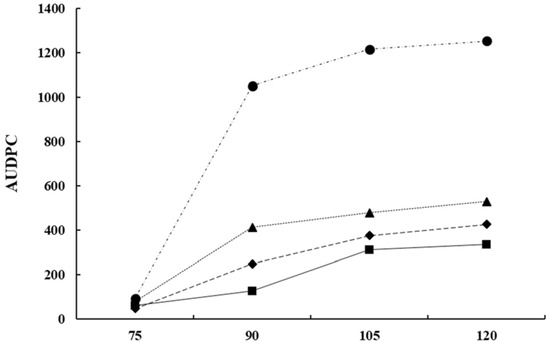
Figure 54. The population of B. subtilis Ctpx-S1 ; Ctpx-S2 and Ctpx-Z3 recovered from the phyllosphere of lupin cultivar I-451 Guaranguito during the 2015 and 2019 growing seasons. First spray at disease onset and fortnightly for eight weeks. Colony counted and results were expressed as CFU B subtilis per gram of lupin leaves. To improve the homogeneity of variances, data of bacterial concentration were log 10-transformed (log10 CFU/g). Each point represents the mean ± SD of four independent repetitions of 10 g leaves. Source: [99].
The total viable epiphytic population on the phylloplane can be determined by a serial dilution plating method considering that bacteria can colonize stomata, trichomes, vein endings, cell wall junctions or even they could be beneath the leaf cuticle [104]. In the work of Falconi et al. [99], lupin stems and leaves sprayed with bacterial antagonists were consistently protected from natural infection of C. lupini fungi. Control was evident in a significant reduction in the AUDPC and suggests that native B. subtilis are more likely the cause of reduction in anthracnose severity. In addition, plants release organic compounds, such as sugars, organic acids, and growth regulators [100] that can stabilize the cell concentration of B. subtilis due to nutrient availability [103]. Thus, when bacterial antagonists adhered, invaded, and survived on flowers, they effectively prevented the pathogen from colonizing the flowers [104]. The addition of 0.5% unrefined sugar to B. subtilis suspensions provides an initial food supply, and increases adherence and colonization of bacteria in cocoa pods, improving monilia pod rot control [105, 106]. Other studies show that a single inoculation of B. pumilus reduced banana sigatoka, Mycosphaerella fijiensis, by 33.6% and delayed the progress of the disease by 21-28 days, compared with the control [107]. Lower AUDPC values in comparison with the control indicate a reduction of the initial inoculum or reduction of the pathogen on the plant [107]. By associating prior findings [80, 82, 83] with the field observations where infections and disease severity were reduced, it can be speculated that biocontrol by native B subtilis resulted not only from the damage of cellular structures (hyphae, mycelium, spores) of the pathogen, as occurred in the in vitro studies, but also by induction of systemic resistance (ISR) and promotion of plant growth mechanism (PGM). An innovative strategy for the integrated management of anthracnose in lupin could involve the activation of ISR by B. subtilis. Future studies could demonstrate more efficient use of these native strains and perhaps allow the establishment of biocontrol strategies.
Table 3. Fungicides and bioproducts reported for the control of lupin anthracnose
|
Active ingredient |
Chemical or biological group |
Target site* |
FRAC code** |
References |
|||
|
Azoxistrobin |
QoI-fungicides |
Complex III: cytochrome bc1 |
C3/11 |
[108, 109] |
|||
|
Iprodione |
dicarboximides |
MAP/Histidine-Kinase in osmotic signal transduction |
E3/2 |
[108, 110] |
|||
|
Procymidone |
dicarboximides |
MAP/Histidine-Kinase in osmotic signal transduction |
E3/2 |
[111] |
|||
|
Carbendazim |
benzimidazoles |
ß-tubulin assembly |
B1/1 |
[111] |
|||
|
Difeconazole |
triazoles |
C14- demethylase in sterol biosynthesis |
G1/3 |
[109] |
|||
|
Copper |
inorganic |
Multi-site contact activity |
M 01 |
[108] |
|||
|
Mancozeb, Maneb |
dithiocarbamates |
Multi-site contact activity |
M 03 |
[108, 109, 110] |
|||
|
Captan |
phthalimides |
Multi-site contact activity |
M 04 |
[110] |
|||
|
Folpet |
chloronitriles |
Multi-site contact activity |
M 05 |
[110] |
|||
|
Clorotalonil |
chloronitriles |
Multi-site contact activity |
M 05 |
[108, 109] |
|||
|
Dithianon |
quinones |
Multi-site contact activity |
M 05 |
[108, 109] |
|||
|
Ciprodinil + Fludioxonil |
anilino-pyrimidines + phenylpyrroles |
Multi-site contact activity |
M 09 |
[110] |
|||
|
Fenhexamid + Tebuconazole |
hydroxyanilides+ triazoles |
3-keto reductase, C4- de-methylation + C14- demethylase in sterol biosynthesis |
G3/17 + G1/3 |
[112] |
|||
|
Pyraclostrobin + Boscalid |
methoxy-carbamates + pyridine-carboxamides |
Complex III: cytochrome bc1 at Qo site + complex II:succinate- dehydrogenase |
C3/11 + C2/7 |
[38] |
|||
|
Azoxystrobin + Difeconazole |
QoI-fungicides + triazoles |
Complex III: cytochrome bc1 at Qo site + C14- demethylase in sterol biosynthesis |
C3/11 + G1/3 |
[38] |
|||
|
B. subtilis |
microbial |
Multiple effects antibiosis, membrane disruption by fungicidal lipopeptides, lytic enzymes, induced plant defense |
BM 02 |
[83, 95, 113]
|
|||
|
*Biochemical mode of action given by the Fungicide Resistance Action Committee (FRAC). Fungicides groups are classified according to their risk level of developing resistance [114]. **Numbers and letters are used to distinguish the fungicide or biological groups according to their cross-resistance behavior. FRAC Codes are organized by numbers and letters [114] |
|||||||
4.2. Chemical control
As there are no resistant varieties of Andean lupin and in the absence of any accurate method of controlling the disease, chemical control has been recommended as the most effective measure to reduce the spread of the disease. Synthetic fungicides reported for the control of lupin anthracnose in the Andean zone and around the world are listed in Table 4. Seed treatment with fungicides reduces transmission of the pathogen from seed coat to seedling, but they do not provide complete control [108]. The synthetic fungicides generally recommended for controlling anthracnose disease are based on copper compounds, di-thiocarbamates, benzimidazole and triazole compounds, manganese ethylenebisdithio-carbamate (Maneb) [108, 109, 111], and carbendazim [111], though the use of fungicides has been found ineffective under severe disease outbreak [108]. Newer chemicals like strobilurins (e.g., azoxystrobin, pyraclostrobin) have also been used for lupin anthracnose management [38, 108, 109]. Different classes of fungicides have specific modes of action and also differ in the duration of disease control. Farmers should take prevailing environmental conditions into consideration in their choice of fungicides [115]. Rotation of two or more different classes of fungicides is highly recommended for increasing the chance of better protection against the disease in the fields [112, 116]. Applications of Bos-calid + Piraclostrobin or Azoxystrobin + Difeconazole significantly reduced the severity and infection in seed and significantly increased grain production [38].
Effective control through the use of chemical fungicides is possible by the timely application during the critical period favorable for the onset of the disease [108, 117]. Generally, fungicides should be applied for seed disinfection [108], at young seedling emergence, including early flowering or full bloom, and early pod formation to restrict the entry of the pathogen to the plant system [108, 109, 111].
Rotating fungicides with different FRAC codes can help delay the development of lupin anthracnose resistance to fungicides. Code numbers on fungicide labels, called “FRAC” groups, can help to develop chemical rotations that delay fungicide resistance (Table 4). The optimal application time is when C. lupini population is small and contained or it could also be when weather conditions make the pathogen susceptible to fungicide, or when lupin is at the most susceptible growth stage (Fig. 6). B. subtilis
The area under disease fpror control of lupin gress curve (AUDPC) of anthracnose is also listed in Table 4. Application of sus-pensions based oncaused by C. lupini on the Andean lupin susceptible cultivar I.450 Andino. Natural pressure of inoculum represented as the control (Ck ) and the AUDPC by effect of Bacillus subtilisB. subtilis
Ctpx-S1 [80, 82, 99] into a rotation with aCtpx-S2 , and Ctpx-Z3 . AUDPC calculates scores for lupin anthracnose severity on the 1-to-6 scale f[92]. Poolied datar fungicide containing mancozeb, chlorothalonil or azoxystrobin [38, 108, 109] a from the 2015 and 2019 growing seasons at El Chaupi, Cantón Mejía, Ecuador, each point at disease onset plus 90, 105, and 120 days. Source: [88].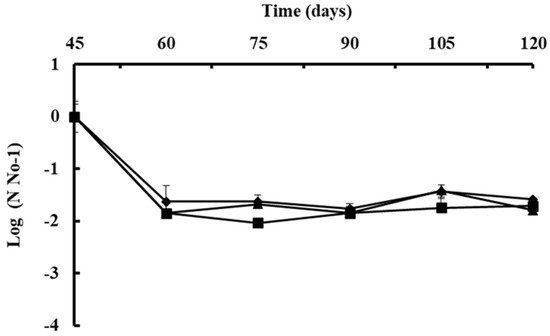
CFU/g). Eachuman health and the environment point represents the mean ± SD of four independent repetitions of 10 g leaves.
Source: [88].
4.2. Chemical Control
| ctive Ingredient | Chemical or Biological Group | Target Site * | FRAC Code ** | References |
|---|---|---|---|---|
| Iprodione | dicarboximides | MAP/Histidine-Kinase in osmotic signal transduction | E3/2 | [99][100] |
| Procymidone | dicarboximides | MAP/Histidine-Kinase in osmotic signal transduction | E3/2 | [99][100] |
| Carbendazim | benzimidazoles | ß-tubulin assembly | B1/1 | [99][100] |
| Difeconazole | triazoles | C14- demethylase in sterol biosynthesis | G1/3 | [99] |
| Copper | inorganic | Multi-site contact activity | M 01 | [99] |
| Mancozeb, Maneb | dithiocarbamates | Multi-site contact activity | M 03 | [99][100][101] |
| Captan | phthalimides | Multi-site contact activity | M 04 | [99] |
| Folpet | chloronitriles | Multi-site contact activity | M 05 | [99] |
| Clorotalonil | chloronitriles | Multi-site contact activity | M 05 | [99][101] |
| Dithianon | quinones | Multi-site contact activity | M 05 | [103][104] |
| Ciprodinil + Fludioxonil |
anilino-pyrimidines + phenylpyrroles | Multi-site contact activity | M 09 | [104] |
| Fenhexamid + Tebuconazole |
hydroxyanilides+ triazoles | 3-keto reductase, C4- de-methylation + C14- demethylase in sterol biosynthesis |
G3/17 + G1/3 |
[104] |
| Azoxistrobin | QoI-fungicides | Complex III: cytochrome bc1 |
C3/11 | [99][100][101] |
| Pyraclostrobin + Boscalid |
methoxy-carbamates + pyridine-carboxamides | Complex III: cytochrome bc1 at Qo site + complex II: succinate-dehydrogenase | C3/11 + C2/7 |
[102] |
| Azoxystrobin + Difeconazole | QoI-fungicides + triazoles |
Complex III: cytochrome bc1 at Qo site + C14- demethylase in sterol biosynthesis | C3/11 + G1/3 |
[102] |
| B. subtilis | microbial | Multiple effects antibiosis, membrane disruption byfungicidal lipopeptides, lytic enzymes, induced plant defense | BM 02 | [38][84][106] |
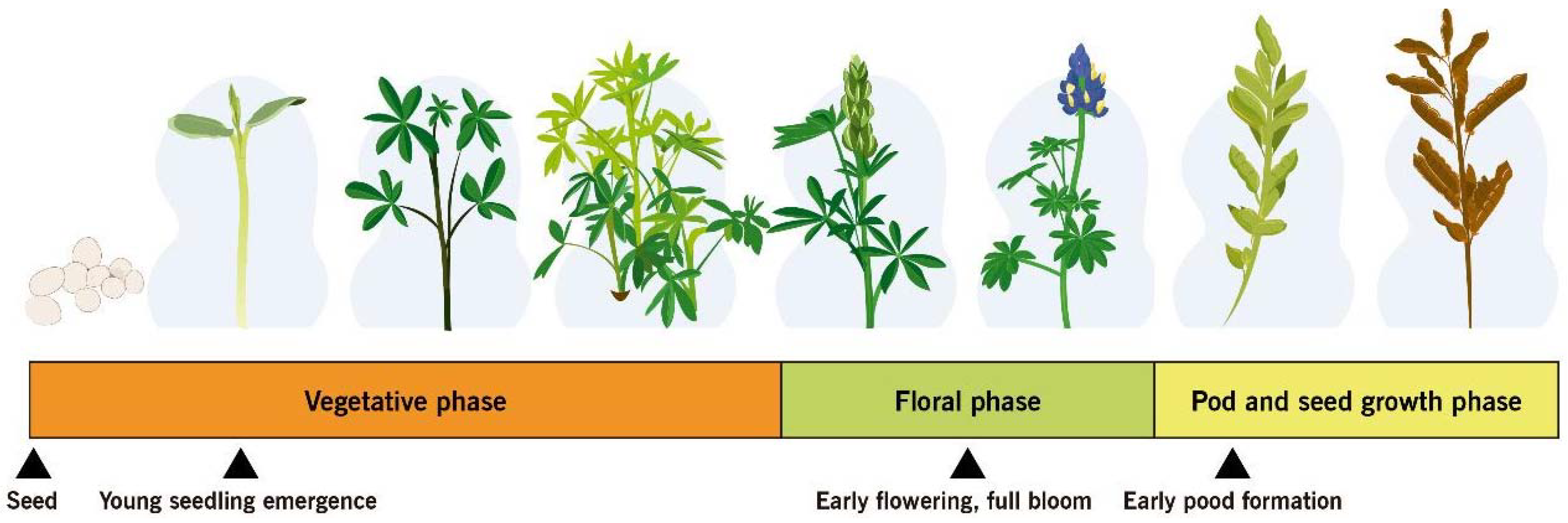
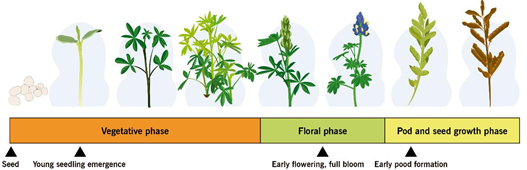
Figure 6. Anthracnose susceptible phenological stages of lupin
4.3. Control volunteer lupins and alternative hosts
Lupin production in the tropical Andean highlands tropical varies in the amount planted, based on that in general follows local rainfall patterns and prevailing prices. The highland tropics are also the home of several alternative hosts for anthracnose, including tamarillo (Solanum betaceum) that are geographically grown in neighboring lands with lu-pin and give the pathogen more opportunities to reproduce and survive [34]. Other studies also showed that C. lupini was able to cross-infect other host plants and Colletotrichum species from soybean also cross-infected lupin with varying degrees of virulence [118]. The plasticity of the interaction of Colletotrichum spp. with different hosts is underline significant. In addition, several species of wild lupin such as lupin afelpado (
4.3. Control Volunteer Lupins and Alternative Hosts
4.4. Ontogenic Resistance
L. alopecuroid-es), lupin rastrero (L. sarmentosus), miniature lupin or allpa chocho in Quechua language (L. microphyllus), Quito´s chocho (L. pubescens) [119] grow in tropical highland areas and thereby support as a reservoir for pathogen growth during drier periods [35]. L. cosentinii can act as another source of infection in Western Australia, where control of naturalized populations is an important component for disease management [13]. In the Andean areas, high levels of inoculum could come from other susceptible cultivated or wild lupins and other alternative hosts in the surrounding areas, consequently, sanitation at the field or farm level is very important for anthracnose management [34].
4.4. Ontogenic resistance
The ability of whole plants or plant parts to resist or tolerate disease as they age and mature is called ontogenic or age-related resistance. Levels of resistance that develop during the aging of plant tissue may greatly reduce eventual disease severity and may even lead to escape from infection or immunity [120]. In lupin, anthracnose resistance is not equally expressed at all developmental stages. An ontogenic model was developed for three lupin species L. angustifolius, L. luteus and L. albus and integrated into a decision support system for anthracnose management. The beginning of flowering and full bloom represent growth stages with high susceptibility [121]. Based on a series of in vivo assays involving Andean lupin genotypes, early cotyledonal stages (2–3leaves) and the beginning of flowering (9–11 leaves ) were susceptible to anthracnose, while the vegetative development stage (6–8 leaves ) was resistant [117]. Cotyledonal and flowering stages of L. mutabilis were confirmed as susceptible to anthracnose through spectroradiometer and unmanned aerial vehicles [121]. This plant response of susceptible genotypes of Andean lupin in Ecuador [3] reflects the current situation of resistant and susceptible cultivars of L. angustifolius in Australia [108, 122], where epidemics of lupin anthracnose progress more slowly in plots of intermediate-aged plants and dramatically increases during flowering. Some stage-specific resistance genes might not be expressed in early and late lupin developmental stages [117]. Targeting disease susceptibility during the life cycle of Andean lupin plant will contribute to the development of a more viable management option for production in areas of high anthracnose risk in Ecuador and maybe to more efficient lupin anthracnose management around the world. Early growth, beginning of flowering, and early pod formation [108, 117, 121, 122] are the stages when lupin is most susceptible to anthracnose. An integrated ontogenic plan can be used during these major plant susceptibility periods for scheduling the application of biological or chemical treatments.
4.5. Integrated methods at critical phenological stages
No single management technique has been found to efficiently control lupin anthracnose, therefore integration of techniques is essential. The first critical step is to reduce infection in seed (Fig. 4) by using dry heat [45], UV-C radiation [51], or UV-B radiation [70]. Further protection can be added by combining physical treatments with other techniques, such as biological [80, 82, 83, 95, 99] or chemical control [38, 108, 109, 110, 111, 112]. If one of the physic treatments of seed is used for high rainfall regions, it should be supplemented with foliar sprays to suppress pathogen dispersion in the field and subsequent infections. Application of fungicides on flowering and podding stages reduced incidence and increased the yield of susceptible and resistant cultivars grown in high-risk areas [108].
Applications of B. subtilis theoretically prevent the establishment of the pathogen, interrupt its development, promote plant growth, and induce acquired resistance in lupin [83]. Because local results obtained under field conditions are consistent [99], bacterial suspensions at 1x10
4.5. Integrated Methods at Critical Phenological Stages
9 UFC/ ml should be applied prior to pathogen infection, at sowing time (Fig. 4). Success in controlling lupin anthracnose disease can be achieved with a sequential two-week application [99]. Shorter interval applications of B. subtilis are often recommended during the rapid growth stages of the plant to obtain a commercially acceptable product [113]. Special attention should be given to cotyledonal, early flowering, full bloom, and pod-filling stages (Fig. 4) because lupin has been shown to be more anthracnose susceptible at these phenological stages [108, 117, 121, 123].
Other technological components are needed to further reduce the use of synthetic pesticides. For this, it is necessary to develop B. subtilis at a semi-industrial or industrial level or to use the pathogen-inhibiting substances extracted from bacteria in rotation plans with chemical products. These rotation plans would include the interaction of B. subtilis-based biopesticides with protective and systemic fungicides to determine dose, frequency, and times of application [124]. A low-cost media for B. subtilis lipopetide production is available [125] and a spray drying formulation [126, 127] has been developed to expand the survival and reproduction of the antagonist under field environmental conditions. A plan that includes the rotation of biological fungicides with chemicals and their application during the most susceptible plant growth stages will greatly minimize infection in the field and reduce losses at harvest for more sustainable production of Andean lupin, until resistant varieties are developed.
5.
The treatment of Andean lupin anthracnose is a complex process due to its multifactorial origins. Protocols of this disease around the world include the use of resistant varieties for higher risk environments, however, resistant varieties are not available for L. mutabilis. Infection-free seed is the first key step to obtaining high production and good quality seed. Thus, seeds should be tested for the presence and quantity of anthracnose infection. Because chemical seed dressing does not guarantee pathogen eradication, and the use of alternative methods, such as dry heat, UV-C and UV-B treatment is more effective for seed disinfection and reducing subsequent potential transmission to seedlings. These methods also induce seed invigoration that translates into positive physiological and biochemical responses in the plant. The use of B. subtilis for seed disinfection is a promising alternative. B. subtilis should be applied to the seed at sowing time.
A second key aspect is to minimize secondary infections and spread of the pathogen in the field. A Combination of alternatives is necessary to protect the crop and reduce yield losses due to anthracnose. Wild lupins and other alternant hosts that grow in surrounding areas of a lupin crop may be common sources of inoculum; if possible these plants should be destroyed. Certain phenological stages of Andean lupin have been identified as susceptible to anthracnose through bioassays and confirmed by using a multispectral lens of an unmanned aerial vehicle camera. It is crucial to reduce the initial processes of the infection cycle by using fungicides, however rotating these with biological methods, such as B. subtilis, may be more effective in delaying the onset and slowing the progress of the disease. The antimicrobial effect of B. subtilis lipopetides may efficiently alter fungal chemical structure enhancing disease control. Eventually, B. subtilis can stimulate plant and root growth, and also turn on resistance genes in the host. B. subtilis should be applied at susceptible phenological stages alone or in a rotation plan with fungicides. Viable cell concentration of B. subtilis can be reduced after application on Andean lupin due to environmental stress, however, native bacterial populations became stable after several applications. New formulations will increase the stability of cells and antimicrobial substances produced by B. subtilis. Future studies on the application, colonization, and survival of native B. subtilis strains on the phylloplane will provide increasingly important components to establish a sustainable program of integrated management of lupin anthracnose. Monitoring of anthracnose and other lupin stresses via geospatial technologies could provide a method to optimize resources for small farmers in underdeveloped regions.
