
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Saburo Matsubara | -- | 2821 | 2022-04-08 09:06:59 | | | |
| 2 | Vicky Zhou | -1 word(s) | 2820 | 2022-04-08 09:38:53 | | |
Video Upload Options
Endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) is widely performed worldwide for various benign and malignant biliary diseases in cases of difficult or unsuccessful endoscopic transpapillary cholangiopancreatography (ERCP). Furthermore, its applicability as primary drainage has also been reported. Although recent advances in EUS systems and equipment have made EUS-HGS easier and safer, the risk of serious adverse events such as bile leak and stent migration still exists. Physicians and assistants need not only sufficient skills and experience in ERCP-related procedures and basic EUS-related procedures such as fine needle aspiration and pancreatic fluid collection drainage, but also knowledge and techniques specific to EUS-HGS.
1. Introduction
2. Step-by-Step Tutorial on EUS-HGS Procedure including Devise Selection
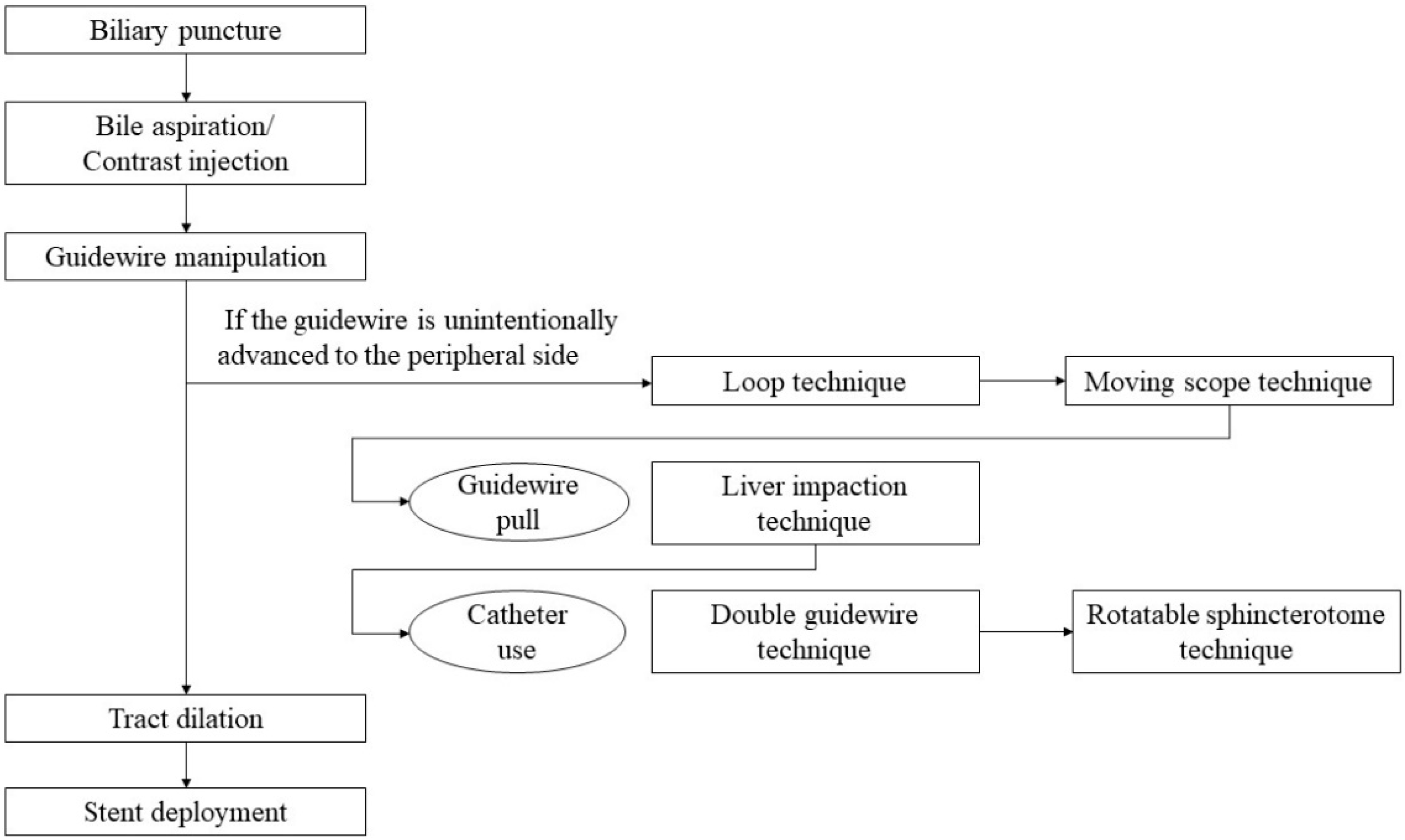
2.1. Selection of Bile Duct Puncture Site and Scope Position
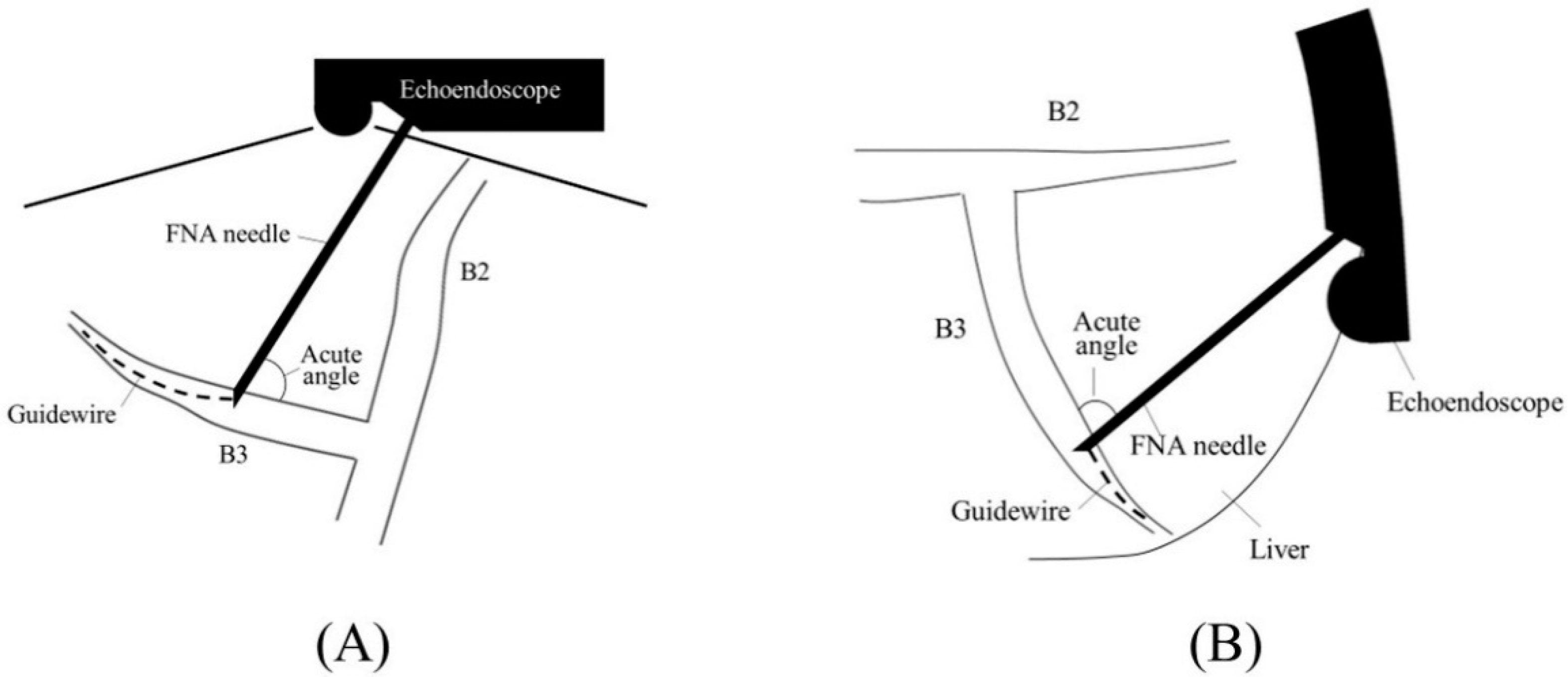
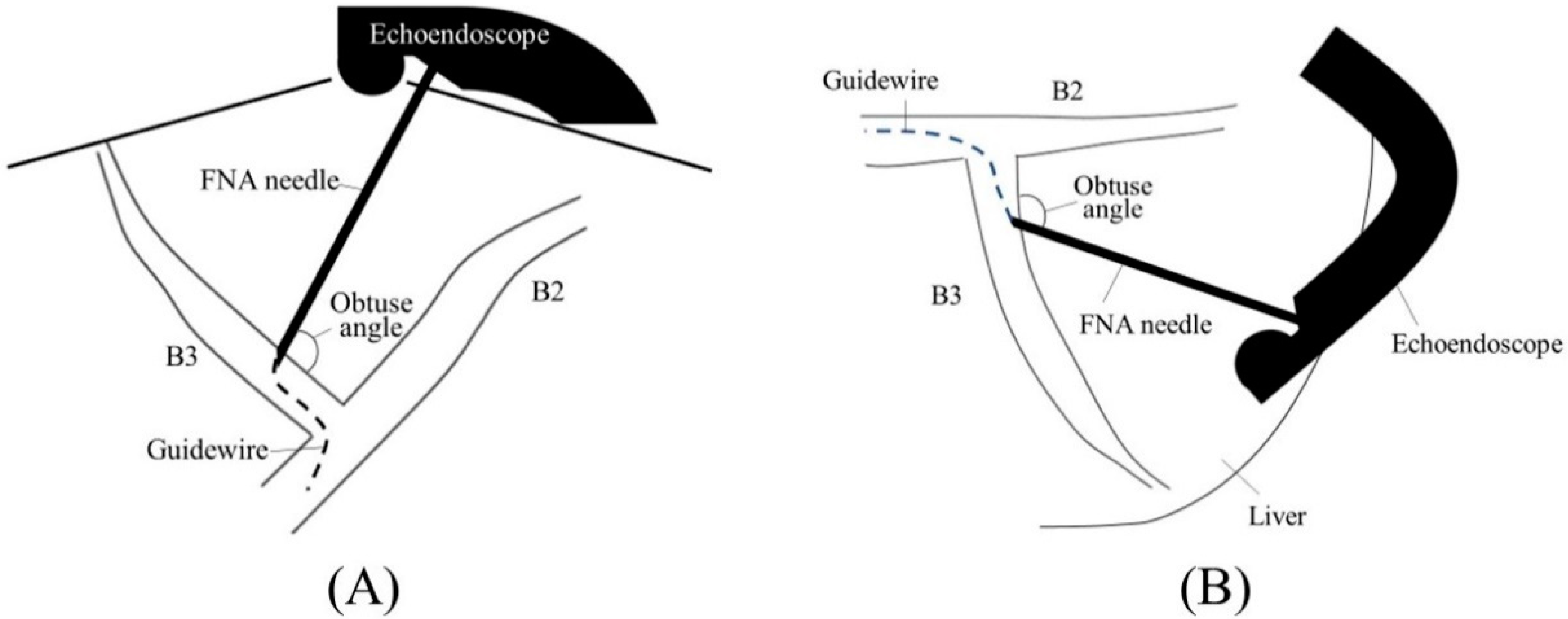

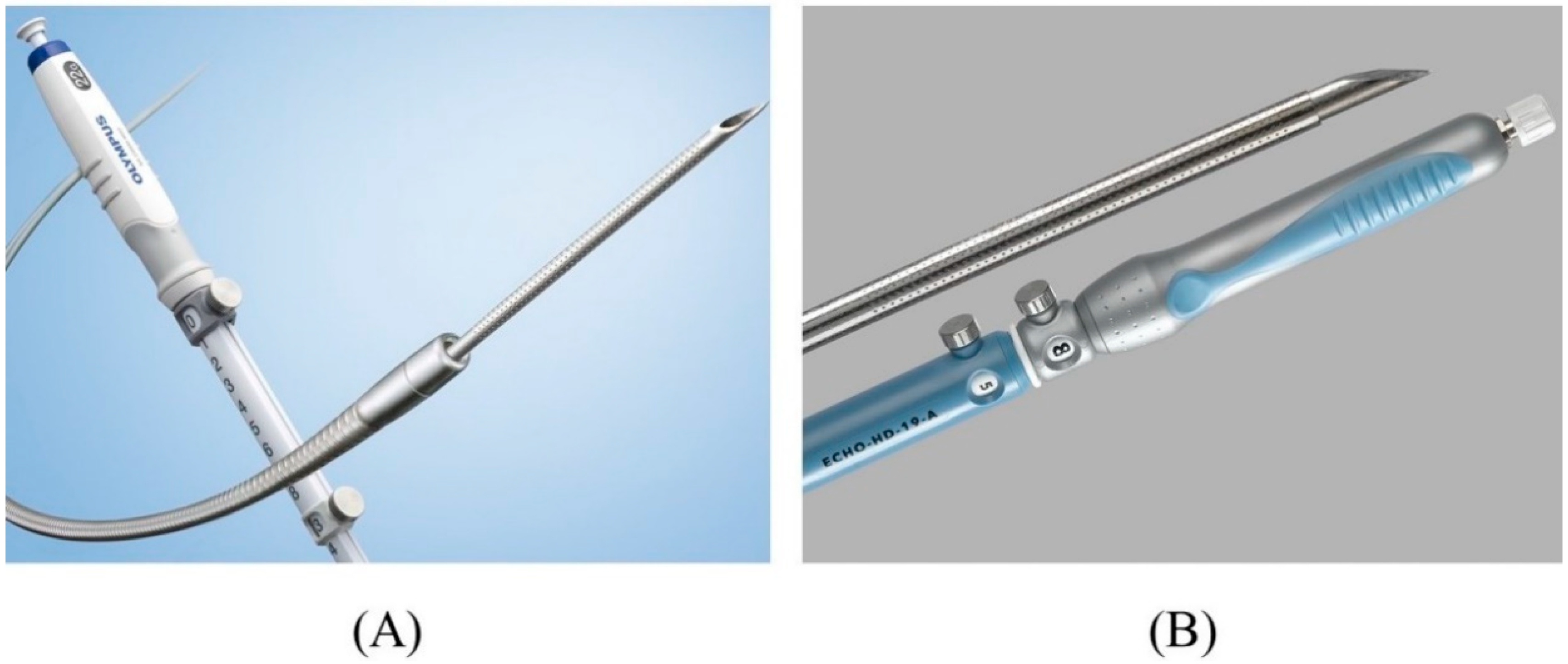
2.2. Biliary Puncture
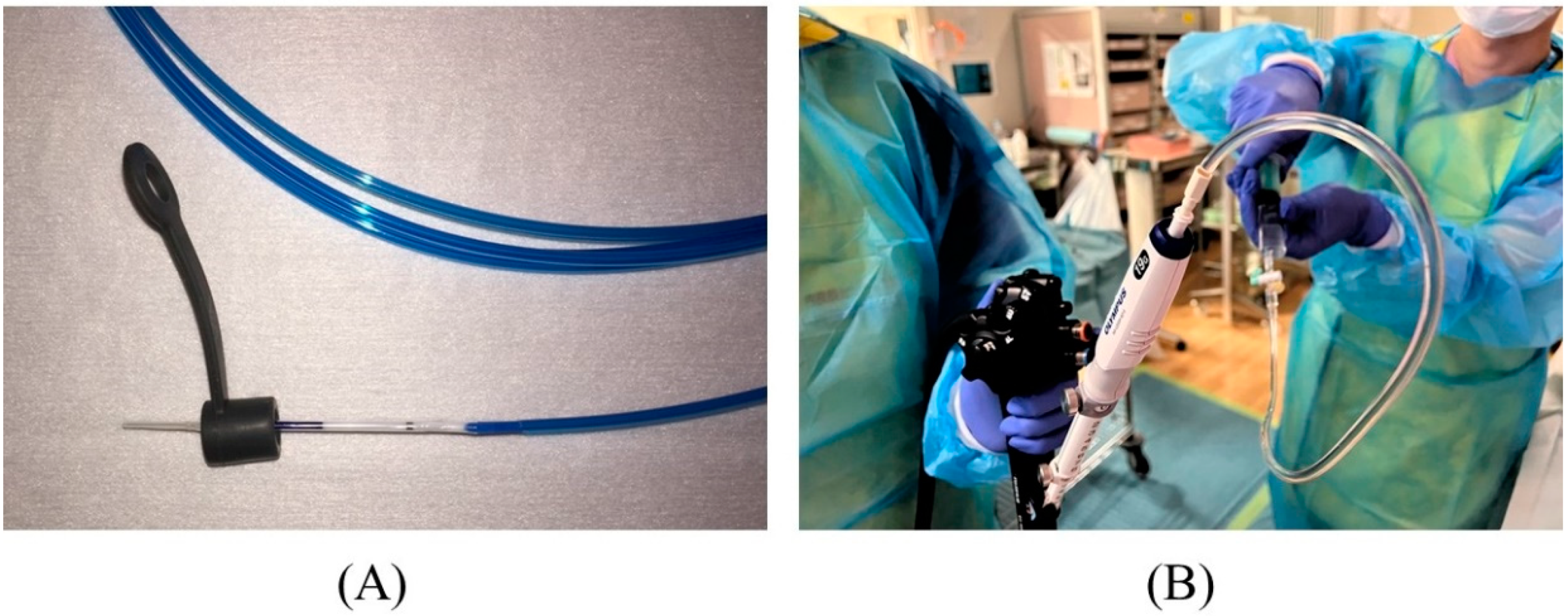
2.3. Contrast Injection
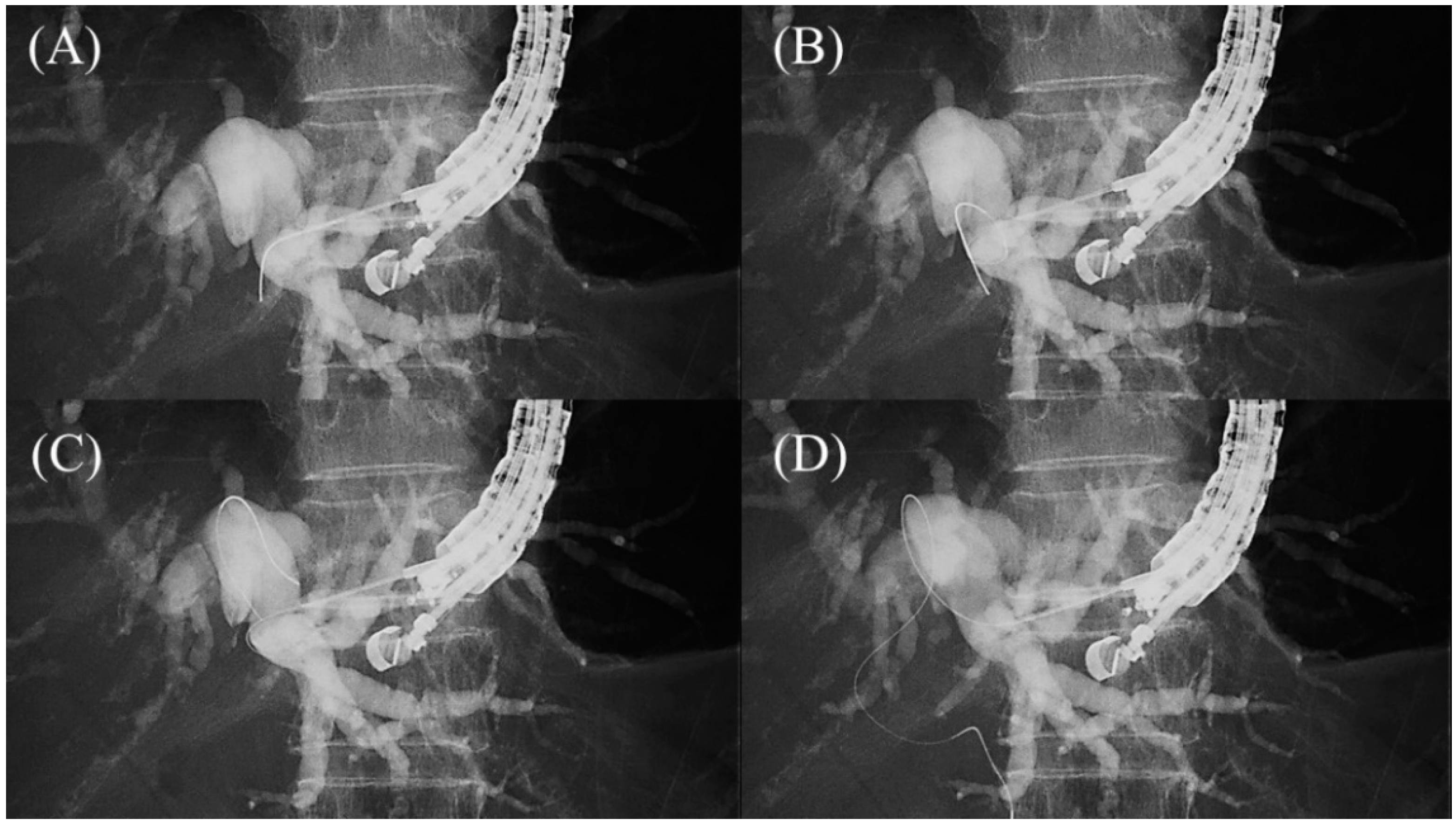
2.4. Guidewire Manipulation
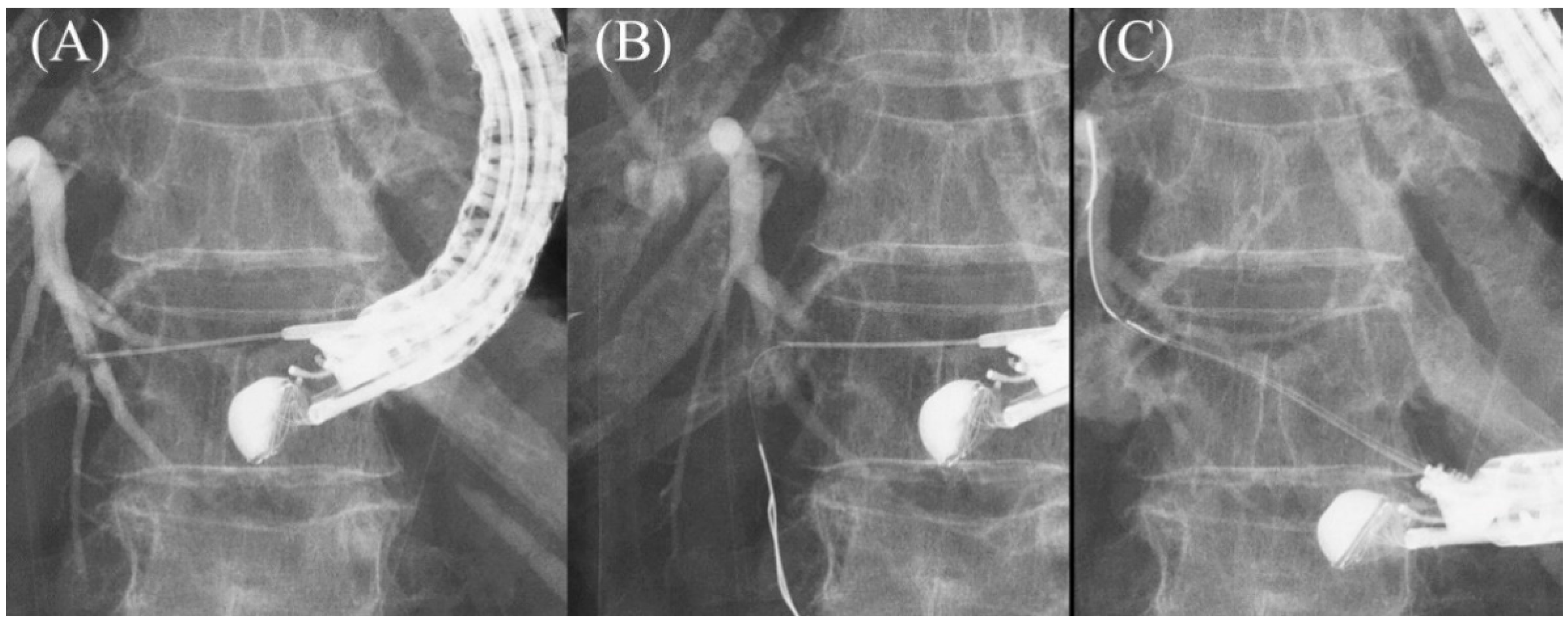
2.5. Tract Dilation
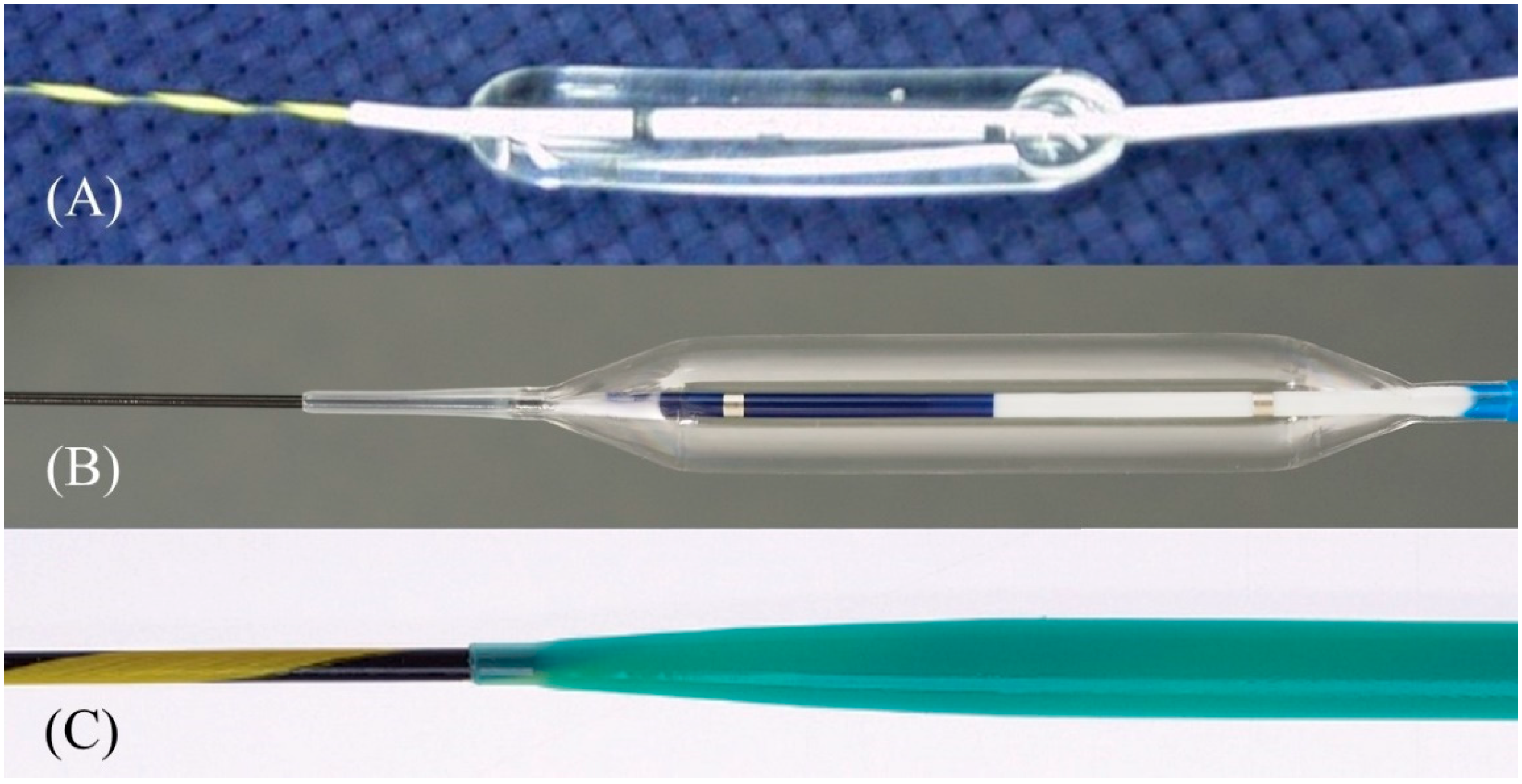
2.6. Stent Deployment
3. Conclusions
References
- Lee, T.H.; Choi, J.H.; Park do, H.; Song, T.J.; Kim, D.U.; Paik, W.H.; Hwangbo, Y.; Lee, S.S.; Seo, D.W.; Lee, S.K.; et al. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin. Gastroenterol. Hepatol. 2016, 14, 1011–1019.e1013.
- Sharaiha, R.Z.; Kumta, N.A.; Desai, A.P.; DeFilippis, E.M.; Gabr, M.; Sarkisian, A.M.; Salgado, S.; Millman, J.; Benvenuto, A.; Cohen, M.; et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg. Endosc. 2016, 30, 5500–5505.
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Tombazzi, C.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914.
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic Ultrasound-Guided Biliary Drainage for Benign Biliary Diseases. Clin. Endosc. 2019, 52, 212–219.
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997.
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282.
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17.
- Isayama, H.; Nakai, Y.; Itoi, T.; Yasuda, I.; Kawakami, H.; Ryozawa, S.; Kitano, M.; Irisawa, A.; Katanuma, A.; Hara, K.; et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J. Hepatobil. Pancreat. Sci. 2019, 26, 249–269.
- Hamada, T.; Isayama, H.; Nakai, Y.; Kogure, H.; Yamamoto, N.; Kawakubo, K.; Takahara, N.; Uchino, R.; Mizuno, S.; Sasaki, T.; et al. Transmural biliary drainage can be an alternative to transpapillary drainage in patients with an indwelling duodenal stent. Dig. Dis. Sci. 2014, 59, 1931–1938.
- Khashab, M.A.; El Zein, M.H.; Sharzehi, K.; Marson, F.P.; Haluszka, O.; Small, A.J.; Nakai, Y.; Park, D.H.; Kunda, R.; Teoh, A.Y.; et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: An international comparative study. Endosc. Int. Open 2016, 4, E1322–E1327.
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic Ultrasound-Guided Biliary Drainage for Unresectable Hilar Malignant Biliary Obstruction. Clin. Endosc. 2019, 52, 220–225.
- Kongkam, P.; Tasneem, A.A.; Rerknimitr, R. Combination of endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography-guided biliary drainage in malignant hilar biliary obstruction. Dig. Endosc. 2019, 31 (Suppl. S1), 50–54.
- Poincloux, L.; Rouquette, O.; Buc, E.; Privat, J.; Pezet, D.; Dapoigny, M.; Bommelaer, G.; Abergel, A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy 2015, 47, 794–801.
- Paik, W.H.; Park, D.H. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, S44–S49.
- Prachayakul, V.; Thamtorawat, S.; Siripipattanamongkol, C.; Thanathanee, P. Bleeding left hepatic artery pseudoaneurysm: A complication of endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2013, 45 (Suppl. S2), E223–E224.
- Martins, F.P.; Rossini, L.G.; Ferrari, A.P. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy 2010, 42 (Suppl. S2), E126–E127.
- Tsujino, T.; Samarasena, J.B.; Chang, K.J. EUS anatomy of the liver segments. Endosc. Ultrasound 2018, 7, 246–251.
- Okuno, N.; Hara, K.; Mizuno, N.; Hijioka, S.; Kuwahara, T.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Risks of transesophageal endoscopic ultrasonography-guided biliary drainage. Int. J. Gastrointest. Interv. 2017, 6, 82–84.
- Ogura, T.; Nishioka, N.; Ueno, S.; Yamada, T.; Yamada, M.; Imoto, A.; Hakoda, A.; Higuchi, K. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2021, 53, 369–375.
- Minaga, K.; Ogura, T.; Shiomi, H.; Imai, H.; Hoki, N.; Takenaka, M.; Nishikiori, H.; Yamashita, Y.; Hisa, T.; Kato, H.; et al. Comparison of the efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: Multicenter, randomized, clinical trial. Dig. Endosc. 2019, 31, 575–582.
- Nakai, Y.; Oyama, H.; Kanai, S.; Noguchi, K.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Hamada, T.; et al. Double Guidewire Technique Using an Uneven Double Lumen Catheter for Endoscopic Ultrasound-Guided Interventions. Dig. Dis. Sci. 2021, 66, 1540–1547.
- Vila, J.J.; Perez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Perez-Millan, A.; Gonzalez-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De la Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141.
- Giovannini, M. EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, S35–S39.
- Committee, A.T.; Hwang, J.H.; Aslanian, H.R.; Thosani, N.; Goodman, A.; Manfredi, M.; Navaneethan, U.; Pannala, R.; Parsi, M.A.; Smith, Z.L.; et al. Devices for use with EUS. VideoGIE 2017, 2, 35–45.
- Ishiwatari, H.; Satoh, T.; Sato, J.; Kaneko, J.; Matsubayashi, H.; Yabuuchi, Y.; Kishida, Y.; Yoshida, M.; Ito, S.; Kawata, N.; et al. Bile aspiration during EUS-guided hepaticogastrostomy is associated with lower risk of postprocedural adverse events: A retrospective single-center study. Surg. Endosc. 2021, 35, 6836–6845.
- Hara, K.; Okuno, N.; Haba, S.; Kuwahara, T.; Koda, H.; Mizuno, N.; Miyano, A. How to perform EUS-guided hepaticogastrostomy easier and safer. J. Hepatobil. Pancreat. Sci. 2020, 27, 563–564.
- Kanno, Y.; Ito, K.; Sakai, T.; Okano, H. Novel combination of a 0.018-inch guidewire, dedicated thin dilator, and 22-gauge needle for EUS-guided hepaticogastrostomy. VideoGIE 2020, 5, 355–358.
- Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Higuchi, K. EUS-guided hepaticogastrostomy for hepaticojejunostomy stricture using a 22G needle and a mechanical dilator (with video). J. Hepatobil. Pancreat. Sci. 2021.
- Ueno, S.; Ogura, T.; Higuchi, K. Moving scope technique for guidewire insertion during endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2021, 33, e109–e110.
- Prachayakul, V.; Aswakul, P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J. Gastroenterol. 2013, 19, 4758–4763.
- Paik, W.H.; Park, D.H.; Choi, J.H.; Choi, J.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Lee, J.B. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J. Gastroenterol. 2014, 20, 5051–5059.
- Park, D.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest. Endosc. 2013, 78, 91–101.
- Amano, M.; Ogura, T.; Onda, S.; Takagi, W.; Sano, T.; Okuda, A.; Miyano, A.; Masuda, D.; Higuchi, K. Prospective clinical study of endoscopic ultrasound-guided biliary drainage using novel balloon catheter (with video). J. Gastroenterol. Hepatol. 2017, 32, 716–720.
- Kanno, Y.; Ito, K.; Koshita, S.; Ogawa, T.; Masu, K.; Masaki, Y.; Noda, Y. Efficacy of a newly developed dilator for endoscopic ultrasound-guided biliary drainage. World J. Gastrointest. Endosc. 2017, 9, 304–309.
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound 2018, 7, 376–382.
- Kawakami, H.; Kubota, Y. Novel wire-guided fine-gauge bougie dilator for transpapillary or endoscopic ultrasonography-guided biliary drainage. Endoscopy 2017, 49, E75–E77.
- Burmester, E.; Niehaus, J.; Leineweber, T.; Huetteroth, T. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest. Endosc. 2003, 57, 246–251.
- Giovannini, M.; Dotti, M.; Bories, E.; Moutardier, V.; Pesenti, C.; Danisi, C.; Delpero, J.R. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy 2003, 35, 1076–1078.
- Ramirez-Luna, M.A.; Tellez-Avila, F.I.; Giovannini, M.; Valdovinos-Andraca, F.; Guerrero-Hernandez, I.; Herrera-Esquivel, J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy 2011, 43, 826–830.
- Attasaranya, S.; Netinasunton, N.; Jongboonyanuparp, T.; Sottisuporn, J.; Witeerungrot, T.; Pirathvisuth, T.; Ovartlarnporn, B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center’s Experience in 31 Cases. Gastroenterol. Res. Pract. 2012, 2012, 680753.
- Fabbri, C.; Luigiano, C.; Fuccio, L.; Polifemo, A.M.; Ferrara, F.; Ghersi, S.; Bassi, M.; Billi, P.; Maimone, A.; Cennamo, V.; et al. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: A case series. Endoscopy 2011, 43, 438–441.
- Artifon, E.L.; Marson, F.P.; Gaidhane, M.; Kahaleh, M.; Otoch, J.P. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc. 2015, 81, 950–959.
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181.
- Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Koshita, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Hashimoto, S.; et al. Metallic stent deployment in endosonography-guided biliary drainage: Long-term follow-up results in patients with bilio-enteric anastomosis. Dig. Endosc. 2012, 24, 457–461.
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291.
- Lee, T.H.; Choi, J.H.; Lee, S.S.; Cho, H.D.; Seo, D.W.; Park, S.H.; Lee, S.K.; Kim, M.H.; Park, D.H. A pilot proof-of-concept study of a modified device for one-step endoscopic ultrasound-guided biliary drainage in a new experimental biliary dilatation animal model. World J. Gastroenterol. 2014, 20, 5859–5866.
- Song, T.J.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2014, 80, 707–711.
- Park, D.H.; Lee, T.H.; Paik, W.H.; Choi, J.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 1461–1466.
- Cho, D.H.; Lee, S.S.; Oh, D.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2017, 85, 1067–1075.
- De Cassan, C.; Bories, E.; Pesenti, C.; Caillol, F.; Godat, S.; Ratone, J.P.; Delpero, J.R.; Ewald, J.; Giovannini, M. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc. Ultrasound 2017, 6, 329–335.
- Leung Ki, E.L.; Napoleon, B. EUS-specific stents: Available designs and probable lacunae. Endosc. Ultrasound 2019, 8, S17–S27.
- Park, S.W.; Lee, S.S. Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? J. Clin. Med. 2020, 9, 3595.
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest. Endosc. 2020, 92, 623–631.e621.
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128.




