Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Melkie Getnet Tadesse | -- | 1782 | 2022-04-07 09:45:31 | | | |
| 2 | Dean Liu | Meta information modification | 1782 | 2022-04-08 04:33:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tadesse, M.; Gebeyehu, E.K.; Adamu, B.; Luebben, J. Polymers-Based Flexible Supercapacitors for Energy. Encyclopedia. Available online: https://encyclopedia.pub/entry/21446 (accessed on 07 February 2026).
Tadesse M, Gebeyehu EK, Adamu B, Luebben J. Polymers-Based Flexible Supercapacitors for Energy. Encyclopedia. Available at: https://encyclopedia.pub/entry/21446. Accessed February 07, 2026.
Tadesse, Melkie, Esubalew Kasaw Gebeyehu, Biruk Adamu, Joern Luebben. "Polymers-Based Flexible Supercapacitors for Energy" Encyclopedia, https://encyclopedia.pub/entry/21446 (accessed February 07, 2026).
Tadesse, M., Gebeyehu, E.K., Adamu, B., & Luebben, J. (2022, April 07). Polymers-Based Flexible Supercapacitors for Energy. In Encyclopedia. https://encyclopedia.pub/entry/21446
Tadesse, Melkie, et al. "Polymers-Based Flexible Supercapacitors for Energy." Encyclopedia. Web. 07 April, 2022.
Copy Citation
Flexible supercapacitors are highly demanding due to their wearability, washability, lightweight property and rollability. Supercapacitors are specially designed capacitors which have huge capacitance value and energy density when compared to the conventional capacitors that are with fast storage ability and high energy density than capacitors
supercapacitor
electrical conductivity
energy storage
banana peel
1. Introduction
Supercapacitors are specially designed capacitors which have huge capacitance value and energy density when compared to the conventional capacitors that are with fast storage ability and high energy density than capacitors [1]. Based on the working and energy storage principle, supercapacitors are categorized into three basic groups as shown in Figure 1. Electric double-layer capacitors (EDLCs) are types of capacitors constructed using three materials the so-called electrodes, electrolytes, and a separator. They are portable, very efficient, and high-power energy storage devices [2]. EDLCs store energy by means of non-faradic principle or electrostatically that encompasses no transmission of charge between the electrolyte and electrode.
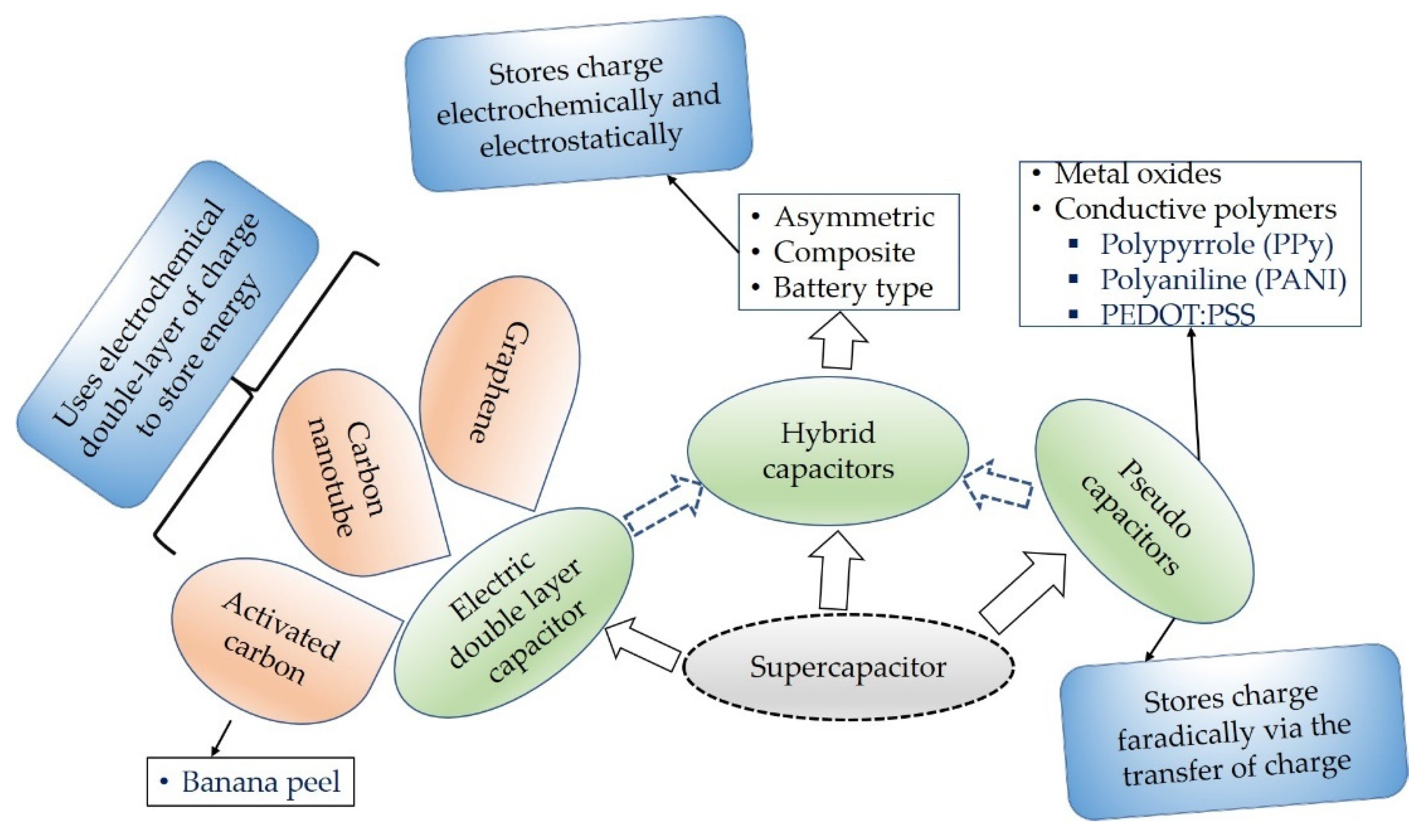
Figure 1. Types of supercapacitors based on its working principles.
Pseudocapacitors on the contrary to EDLCs store charge via the transfer of charge amongst the electrode and electrolyte faradically [3]. Pseudocapacitors employed various conductive polymers such as polypyrrole (PPy) and polyaniline (PANI) [3], poly(3,4-ethylenedioxtthiophne) polystyrene sulfonate (PEDOT:PSS) [4]. Due to the charging and discharging behavior and the reduction-oxidation rection occurred during processing, pseudocapacitors lack stability. Stability against the environment is very crucial for such types of materials.
Hybrid supercapacitors for energy storage principle is based on the combinations of the EDLCs and pseudocapacitors. Hybrid supercapacitors comprise the compensations of pseudocapacitors and EDLCs [5]. The limitations in both supercapacitor types are eliminated in the hybrid system and show better electrochemical characteristics.
2. Supercapacitors Based on Carbonized Banana Peels
Banana peels are conventionally waste materials that habitually discarded after consuming the edible parts. This causes a temporary pollution to the surrounding area where high marketing areas are available. On the other hand, the depletion of fossil fuels along with the dynamic climatic alteration requires extra exploitation of spotless and justifiable energy alternative sources [6]. In addition, the recent surge of flexile and lightweight electronics with high durability pushes most industries to the implementation of the best alternatives to renewable energy sources. Among the flexible and lightweight energy storage devices, supercapacitors are widely researched. Supercapacitors are the most promising energy storage alternatives for short-term applications. Supercapacitors have high power density and fast charge-discharge rate beyond they do have long lasting life cycle against the surrounding environment [7].
Carbon-based porous structure materials have been proven to be promising as flexible, lightweight, and durable supercapacitors [7]. Porous supercapacitors based on carbonized banana peel is one of the promising research areas in recent years [8][9][10][11]. Banana peel can be prepared for a possible supercapacitor in different ways. Figure 2 illustrates one route of manufacturing supercapacitor from carbonized banana peel.

Figure 2. Illustrations showing process of manufacturing flexible supercapacitors starting from raw material preparation, carbonization and supercapacitor assembly.
Banana peel is an excellent potential for manufacturing supercapacitors. Various carbonization methods have been employed to improve the performance of supercapacitors with irrespective of its purposes and achieved high capacitance values. Table 1 summarizes the applied carbonization methods and its effect on the capacitance values.
Table 1. Summary of carbonized supercapacitor (SC) manufacturing based on banana peel.
| Method of Carbonization |
Purposes | Results Achieved | Ref. |
|---|---|---|---|
| One-step chemical activation | Improving electrochemical performance | Capacitance of 227 Fg−1 at 1 Ag−1 | [12] |
| Carbonization without activation | Green and cost-effective facile route | Notable specific capacitance (811 Fg−1) | [9] |
| one-step hydrothermal method | To get excellent electrochemical performance | Capacitance enduring 51 Fg−1 at 5.0 Ag−1 | [13] |
| Chemical activation | To improve conductivity and electrochemical performance | Specific capacitance of 90.23 Fg−1 at 10 mVs−1 |
[14] |
| Heating banana peel soaked with KOH at high temperature | To check stability against multiple electronic cycling and bending | Devices displayed high areal capacitance of 88 mF/cm2 at 10 mV/s scan rate | [15] |
| two-step hydrothermal process | To reach easily to the active site and to shorten the ion transport path | Large capacitance of 816 Fg−1 at the current density of 5 mA cm−2 | [16] |
| Green pyrolysis | To check energy storage ability and environmental remediation | Capacitance of 655 Fg−1 in 1 M at a current density of 0.35 Ag−1 & excellent cyclic stability of 79.3% | [17] |
| Sulfur-doped (chemical carbonization) | Sustainable supercapacitor production | High Brunauer-Emmett-Teller surface area of 2224.9 m2/g, a large pore volume of 0.77 cm3/g | [10] |
| Carbonization with chemical activation | To see relationship of surface area to cell capacitance | SC increased from 59–~265 Fg−1 at 0.1 Ag−1 | [18] |
| Biological activation | Optimization of precursors and synthesis methods | Get specific capacitance of 476 Fg−1 in 1 M H2SO4 electrolyte | [19] |
| Chemical co-precipitation method | To get high electrochemical property | specific capacitance of 465 Fg−1 at a scan rate of 10 mV s−1 by CV | [11] |
| Biological fermentation | Stabilize the structure of electrodes | High-capacity hold of 58.35% after 100 cycles | [20] |
| Hydrothermal method | To increase the electrochemical performance | Had a specific capacitance of 139.6 Fg−1 at 300 mA g−1 | [21] |
| straightforward carbonization | To improve electrochemical performance | Specific capacitance improved from 59 to 258–273 Fg−1 at 0.1 Ag−1 | [22] |
| KOH pellets at different carbonization temperatures | To improve electrochemical performance | Specific capacitance of 165 Fg−1 at energy density of 18.6 Wh kg−1 at 0.5 Ag−1 | [23] |
| Carbonization followed by activation | To see the relationship between surface area and electrochemical property | Surface area had significant effect on electrochemical property (specific capacitance of 68 Fg−1) | [24] |
| H2SO4 activation | Carbon nanofiber synthesis | Carbon nanofiber formed at 700 °C) | [25] |
| KOH | Absorption study | reflection loss peak of −44.59 dB at 10.84 GHz | [26] |
As indicated in Table 1, carbonization of biomass resulted in carbon materials with porous structure and hence carbon materials have used for energy storage materials as supercapacitors [27]. Recent developments in the production of flexible supercapacitors indicated that banana peels represent a possible method to produce durable, flexible, and light weight energy storage devices. Supercapacitors based on banana peel achieved high capacitance value due to the ability of embodying high surface area which in turn can encompass highly porous structures. This most likely proves that carbon materials based on banana peels will continue to strive to be one of the protentional applications for supercapacitor production as far as optimization in terms of activation type, high porosity, and flexibility achieved.
Banana peels (BP) have been used as the supercapacitor electrode after carbonization. They can be carbonized by applying various mechanisms such as chemical activation [28], hydrothermal methods [21], biological fermentation [20], and pyrolysis [17]. For the sake of obtaining higher capacitance value, various chemicals may be employed to chemically activate the banana peel carbon. The chemical activation may increase the surface area of the carbonized banana peel with affecting the structure of activated carbon. Activation helps to increases the surface area to volume ratio and to enhance absorption is enhanced. Moreover, the supercapacitor for chemically-activated banana peel achieved exceptional recurring steadiness with capacitance maintenance of ~97% for 5000 cycles [12]. Banana peel carbon provides the highest performance when activated using chemicals. Furthermore, the chemical precipitation has helped to enhance the supercapactive performance of carbonized supercapacitors, and hence chemical activation in suggested for the production of durable supercapacitors in the future.
3. Supercapacitors Based on PEDOT:PSS
3.1. Introduction
Poly(3,4-ethylenedioxythiophne)-polystyrene sulfonate (PEDOT:PSS) is the most recently explored, widely used, and successfully implemented intrinsically conductive polymers. The utmost plausible reason for this is due to excellent electromechanical performance [29], high conductivity and wash durability [30], and water processability and light transmissive behaviour [31][32]. In addition to this point, PEDOT:PSS is stable in environment and can be processed up to high temperature ranges. PEDOT:PSS alone is not good conductor as hydrophilic PSS hides the conductivity. In this case, some researchers have been using polyethylene glycol (PEG) [23], dimethyl sulfoxide (DMSO) [24], and ethylene glycol (EG) [25] to enhance the conductivity. When PEDOT:PSS is doped with these solutions, structural rearrangement has taken place (Figure 3c) [30].
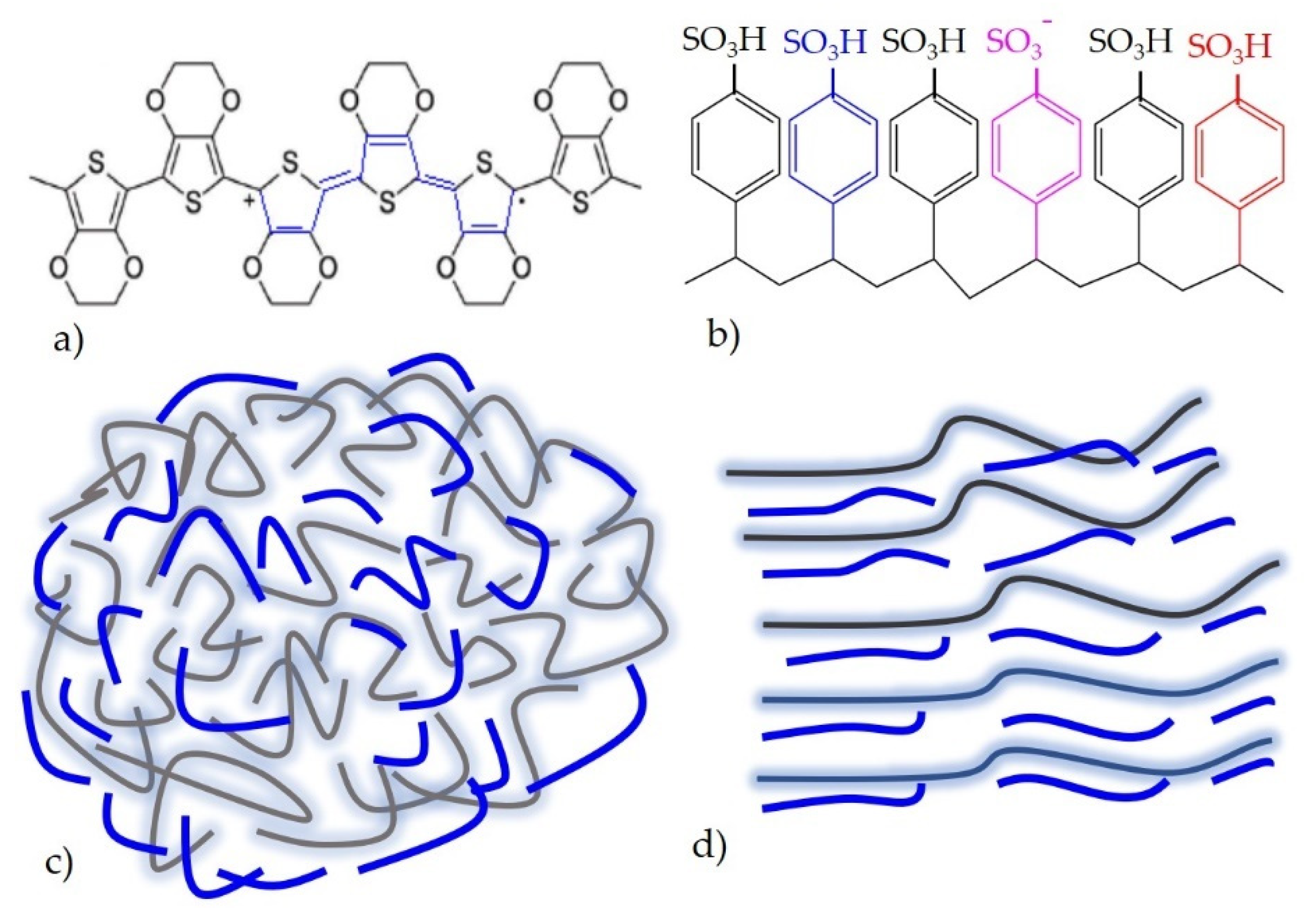
Figure 3. (a) Chemical structure of PEDOT; (b) chemical structure of PSS; (c) when PSS is added to PEDOT; a water dispersion solution is created; (d) when PEDOT:PSS is doped with conductive enhancers, a structural re-arrangement is formed, adapted from Ref. [30].
Perhaps the most well-known example of an organic intrinsic semiconductor polymer is the polymer poly(3,4-ethylenedioxythiophene) (PEDOT), predominantly when it intricates with poly(styrene sulfonate-PSS) (PEDOT:PSS). PSS surrounds and mostly found on the surface of PEDOT and which helps PEDOT to be dispersible in water. It is extremely conductive, light-transmissive to a significant extent, water-processable, and highly flexible. Considerable recent research on this ubiquitous material has focused on enhancing its deformability beyond flexibility (a property that any suitably thin material possesses) to stretchability (a property that requires molecular or nanoscale structure engineering). The aforementioned properties of PEDOT:PSS are fundamental, which may help researchers to investigate the use of PEDOT:PSS as a durable energy storage materials such as supercapacitors.
Poly(3,4-ethylenedioxythiphene)-poly(styrene sulfonate) has been used in the production of high-performance supercapacitors. Table 2 summarizes the use of PEDOT:PSS to produce supercapacitor and its efficiency. Using PEDOT:PSS conductive polymer not only brought electrochemical performance but also possess excellent durability against washing [29].
Table 2. Summary of supercapacitors based on PEDOT:PSS with capacitance (C) main factor.
| Precursor/Composite | Capacitance | Performance | Ref. |
|---|---|---|---|
| rGO | 226.5 F cm−3/279.3 mF cm−2 at 0.5 A cm−3 | 74.7% C retention at 50 A cm−3 | [33] |
| WO3 | 1139.6 mF cm−2 at 2 mA cm−2 | Working voltage of 1.6 V | [34] |
| Cellulose nanofibrils | 854.4 mF cm−2 at 5 mV/s | Areal ED of 30.86 μWh cm−2 | [35] |
| PANI, PPy | 156 mF cm−2 C at 1 mA cm−2 CD | 41% capacity persisted at 20 mA cm−2 | [36] |
| MgTf2 | 280 Fg−1 at 3 mV/s and 376.6 Fg−1 at 100 mA g−1 | PD ~100.08 Wkg−1 | [37] |
| Graphene | C of 2 mF cm−2 at a scan rate of 102 mV s−1 | >95% C retaining after 103 cycles | [38] |
| Polypyrrole | 12.4–10.5 F cm−3 at a CD of 40–320 mA cm−3 | C retention rate of 88.1% for 103 charges/discharge cycles | [39] |
| Carbon nanofibers | C of 1321 Fg−1, at a scanning speed of 1 mV/s | Retention of 80% of its performance after 2500 CV cycles | [40] |
| MnO2 microspheres | Capacitance of 135.4 mF cm−2 | 94% C maintenance after 3000 cycles | [41] |
| rGO/CoFe2O4 | Capacitance of 229.6 mF cm−2 | ED and PD of 25.9 Wh kg−1 and 135.3 W kg−1, respectively | [42] |
| Poly(acrylamide) | specific C of 327 Fg−1 at 3 mV/s | highest ionic conductivity of 13.7 × 10−3 S/cm at 22 ± 2 °C | [43] |
| CoCCHH-CoSe | C of 440.6 Fg−1 at 1 Ag−1 | ED of 137.7 Wh kg−1 | [44] |
| PANI Nanofiber | C of 301.71 mF cm−2 at CD of 1 mA cm−2 | ED of 0.023 mWh cm−2, with PD of 0.279 mW cm−2 at a lower current density of 1 mA cm−2 | [45] |
| nanoflower MnOx | C of 580 mF·cm−2 at 0.5 mA | >90% for 40% stretch | [46] |
| ---- | C of 3.92 mF/cm2 at 1 mA/cm2 | C retention > 90% after 3 × 103 cycles | [47] |
| ---- | Capacitance of 990 mF cm−2 | C retention of 74.7% after 14,000 cycles | [48] |
| Alginate/PPy | Capacitance of 246.4 mF cm−2 | 97% of initial values after 180° bending | [49] |
| Ag-coated Tyvek | Mass C (138.7 Fg−1) & volume C (544.2 F/cm3) at the scan rate of 50 mV/s. | 91.2% retention after 100 cycles | [50] |
| PVA/H2SO4 | Areal C of 44.5 mF cm−2 at PD of 0.04 mW cm−2 | 92% retention at 200% stretchability | [51] |
| MWCNT | specific capacitance of 235 Fg−1 at 5 mV s−1 | retention of about 92% in 1M H2SO4 electrolyte | [52] |
GO, reduced graphene oxide; WO3, tungsten trioxide; MgTf2, magnesium trifluoromethanesulfonate; CD, current density; PD, power density; ED, energy density; CoCCHH-CoSe, heterogenous tube; MWCNT, multi-walled carbon nanotubes.
References
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473.
- Skinner, B.; Chen, T.; Loth, M.S.; Shklovskii, B.I. Theory of volumetric capacitance of an electric double-layer supercapacitor. Phys. Rev. E 2011, 83, 056102.
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527.
- Cheng, T.; Zhang, Y.-Z.; Zhang, J.-D.; Lai, W.-Y.; Huang, W. High-performance free-standing PEDOT:PSS electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499.
- Yi, R.; Chen, S.; Song, J.; Gordin, M.L.; Manivannan, A.; Wang, D. High-performance hybrid supercapacitor enabled by a high-rate Si-based anode. Adv. Funct. Mater. 2014, 24, 7433–7439.
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266.
- Wu, Q.; He, T.; Zhang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Zhao, L.; Wu, Y.; Ran, F. Cyclic stability of supercapacitors: Materials, energy storage mechanism, test methods, and device. J. Mater. Chem. A 2021, 9, 24094–24147.
- Cárdenas-Martínez, J.; España-Sánchez, B.L.; Esparza, R.; Ávila-Niño, J.A. Flexible and transparent supercapacitors using electrospun PEDOT:PSS electrodes. Synth. Met. 2020, 267, 116436.
- Al Kiey, S.A.; Hasanin, M.S. Green and facile synthesis of nickel oxide-porous carbon composite as improved electrochemical electrodes for supercapacitor application from banana peel waste. Environ. Sci. Pollut. Res. 2021, 28, 66888–66900.
- Chen, H.; Zhao, Z.; Qi, P.; Wang, G.; Shi, L.; Yu, F. Sulphur-doped banana peel-derived activated carbon as electrode materials for supercapacitors. Int. J. Nanomanuf. 2019, 15, 181–195.
- Kaushal, I.; Maken, S.; Sharma, A.K. SnO2 mixed banana peel derived biochar composite for supercapacitor application. Korean Chem. Eng. Res. 2018, 56, 694–704.
- Tripathy, A.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J. Environ. Chem. Eng. 2021, 9, 106398.
- Raji, A.; Thomas Nesakumar, J.I.E.; Mani, S.; Perumal, S.; Rajangam, V.; Thirunavukkarasu, S.; Lee, Y.R. Biowaste-originated heteroatom-doped porous carbonaceous material for electrochemical energy storage application. J. Ind. Eng. Chem. 2021, 98, 308–317.
- Kandasamy, S.K.; Arumugam, C.; Sajitha, A.S.; Rao, S.P.; Selvaraj, S.; Vetrivel, R.; Selvarajan, R.; Alosaimi, A.M.; Khan, A.; Hussein, M.A.; et al. Paradisiaca/Solanum Tuberosum Biowaste Composited with Graphene Oxide for Flexible Supercapacitor. J. New Mater. Electrochem. Syst. 2021, 24, 21–28.
- Singh, A.; Ghosh, K.; Kumar, S.; Agarwal, A.K.; Jassal, M.; Goswami, P.; Chaturvedi, H. Flexible planar asymmetric supercapacitor using synthesized few-layer graphene and activated carbon from biomass for wearable energy storage. Nanotechnol. Percept. 2019, 15, 183–188.
- Ren, B.; Fan, M.; Yang, X.; Wang, L.; Yu, H. 3D Hierarchical structure Electrodes of MnO2 Nanosheets Decorated on Needle-like NiCo2O4 Nanocones on Ni Foam as a cathode material for Asymmetric Supercapacitors. ChemistrySelect 2019, 4, 5641–5650.
- Kaushal, I.; Saharan, P.; Kumar, V.; Sharma, A.K.; Umar, A. Superb sono-adsorption and energy storage potential of multifunctional Ag-Biochar composite. J. Alloys Compd. 2019, 785, 240–249.
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Awitdrus; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H.; et al. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor aplication. Proc. J. Phys. Conf. Ser. 2008, 1116, 032040.
- Lian, Y.M.; Ni, M.; Zhou, L.; Chen, R.J.; Yang, W. Synthesis of Biomass-Derived Carbon Induced by Cellular Respiration in Yeast for Supercapacitor Applications. Chem.-A Eur. J. 2018, 24, 18068–18074.
- Xia, L.; Zhou, Y.; Ren, J.; Wu, H.; Lin, D.; Xie, F.; Jie, W.; Lam, K.H.; Xu, C.; Zheng, Q. An Eco-friendly Microorganism Method to Activate Biomass for Cathode Materials for High-Performance Lithium-Sulfur Batteries. Energy Fuels 2018, 32, 9997–10007.
- Yang, G.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367.
- Wang, Q.; Zhou, M.; Zhang, Y.; Liu, M.; Xiong, W.; Liu, S. Large surface area porous carbon materials synthesized by direct carbonization of banana peel and citrate salts for use as high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 4294–4300.
- Fasakin, O.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Oyedotun, K.O.; Eleruja, M.A.; Manyala, N. Synthesis and characterization of porous carbon derived from activated banana peels with hierarchical porosity for improved electrochemical performance. Electrochim. Acta 2018, 262, 187–196.
- Taer, E.; Taslim, R.; Aini, Z.; Hartati, S.D.; Mustika, W.S. Activated carbon electrode from banana-peel waste for supercapacitor applications. Proc. AIP Conf. Proc. 2017, 1801, 040004.
- Sari, S.N.; Melati, A. Facile preparation of carbon nanofiber from banana peel waste. Mater. Today Proc. 2019, 13, 165–168.
- Yusuf, J.Y.; Soleimani, H.; Chuan, L.K.; Sanusi, Y.K.; Adebayo, L.L. Physicochemical properties and microwave absorption performance of Co3O4 and banana peel-derived porous activated carbon composite at X-band frequency. J. Alloys Compd. 2021, 888.
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sour. 2010, 195, 7880–7903.
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217.
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT:PSS and polyurethane. J. Mater. Sci. 2019, 54, 9591–9602.
- Tadesse, M.G.; Loghin, C.; Chen, Y.; Wang, L.; Catalin, D.; Nierstrasz, V. Effect of liquid immersion of PEDOT: PSS-coated polyester fabric on surface resistance and wettability. Smart Mater. Struct. 2017, 26, 065016.
- Kayser, L.V.; Lipomi, D.J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133.
- Tadesse, M.G.; Dumitrescu, D.; Loghin, C.; Chen, Y.; Wang, L.; Nierstrasz, V. 3D Printing of NinjaFlex Filament onto PEDOT:PSS-Coated Textile Fabrics for Electroluminescence Applications. J. Electron. Mater. 2018, 47, 2082–2092.
- Teng, W.; Zhou, Q.; Wang, X.; Gao, J.; Hu, P.; Du, Y.; Li, H.; Wang, J. Enhancing ions/electrons dual transport in rGO/PEDOT: PSS fiber for high-performance supercapaciton. Carbon 2022, 189, 284–292.
- He, Y.; Liang, A.; Zhu, D.; Hu, M.; Xu, L.; Chao, S.; Zhou, W.; Wu, Y.; Xu, J.; Zhao, F. Organic-inorganic hybrid electrode engineering for high-performance asymmetric supercapacitor based on WO3-CeO2 nanowires with oxygen vacancies. Appl. Surf. Sci. 2022, 573, 151624.
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT:PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem. Eng. J 2022, 428, 131994.
- Lai, H.; Bai, C.; Wang, Y.; Fan, Z.; Yuan, Y.; Jiao, H. Highly Crosslinked Conductive Polymer Nanofibrous Films for High-Rate Solid-State Supercapacitors and Electromagnetic Interference Shielding. Adv. Mater. Interfaces 2022, 9, 2102115.
- Bashir, S.; Hina, M.; Iqbal, J.; Jafer, R.; Ramesh, S.; Ramesh, K. Self-healable poly (N, N-dimethylacrylamide)/poly (3, 4-ethylenedioxythiophene) polystyrene sulfonate composite hydrogel electrolytes for aqueous supercapacitors. J. Ener. Storage 2022, 45, 103760.
- Li, Z.; Ruiz, V.; Mishukova, V.; Wan, Q.; Liu, H.; Xue, H.; Gao, Y.; Cao, G.; Li, Y.; Zhuang, X.; et al. Inkjet Printed Disposable High-Rate On-Paper Microsupercapacitors. Adv. Funct. Mater. 2022, 32, 2108773.
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E.; Wu, H.; Guo, S. Robust quasi-solid-state integrated asymmetric flexible supercapacitors with interchangeable positive and negative electrode based on all-conducting-polymer electrodes. J. Alloys Compd. 2021, 887, 161362.
- Altin, Y.; Celik Bedeloglu, A. Poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate-coated carbon nanofiber electrodes via dip-coating method for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2021, 32, 28234–28244.
- Li, D.; Yang, S.; Chen, X.; Lai, W.Y.; Huang, W. 3D Wearable Fabric-Based Micro-Supercapacitors with Ultra-High Areal Capacitance. Adv. Funct. Mater. 2021, 31, 2107484.
- Song, J.; Li, W.; Xin, J.; Wang, W.; Song, K.; Chen, X.; Yin, G. The continuous porous PEDOT:PSS film improves wettability and flexibility of the rGO/CoFe2O4 paper electrodes for symmetric supercapacitors. Appl. Surf. Sci. 2021, 568, 150915.
- Hina, M.; Bashir, S.; Kamran, K.; Iqbal, J.; Ramesh, S.; Ramesh, K. Fabrication of aqueous solid-state symmetric supercapacitors based on self-healable poly (acrylamide)/PEDOT:PSS composite hydrogel electrolytes. Mater. Chem. Phys. 2021, 273, 125125.
- Song, J.; Li, W.; Song, K.; Qin, C.; Chen, X.; Sui, Y.; Zhao, Q.; Ye, Y. Synergistic effect of defects and porous structure in CoCCHH-CoSe heterogeneous-tube @PEDOT:PSS foam towards elastic supercapacitor with enhanced pseudocapacitances. J. Colloid Interface Sci. 2021, 602, 251–260.
- Zhang, X.; Wang, T.; Li, S.; Shen, X. Electrodeposition Polyaniline Nanofiber on the PEDOT:PSS-Coated SiNWs for High Performance Supercapacitors. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4260–4271.
- Pullanchiyodan, A.; Manjakkal, L.; Ntagios, M.; Dahiya, R. MnO x-Electrodeposited Fabric-Based Stretchable Supercapacitors with Intrinsic Strain Sensing. ACS Appl. Mater. Interfaces 2021, 13, 47581–47592.
- Guan, X.; Pan, L.; Fan, Z. Flexible, transparent and highly conductive polymer film electrodes for all-solid-state transparent supercapacitor applications. Membranes 2021, 11, 788.
- Yang, J.; Cao, Q.; Tang, X.; Du, J.; Yu, T.; Xu, X.; Cai, D.; Guan, C.; Huang, W. 3D-Printed highly stretchable conducting polymer electrodes for flexible supercapacitors. J. Mater. Chem. A 2021, 9, 19649–19658.
- Wang, P.; Du, X.; Wang, X.; Zhang, K.; Sun, J.; Chen, Z.; Xia, Y. Integrated fiber electrodes based on marine polysaccharide for ultrahigh-energy-density flexible supercapacitors. J. Power Sour. 2021, 506, 230130.
- Liu, T.; Li, C.; Liu, H.; Zhang, S.; Yang, J.; Zhou, J.; Yu, J.; Ji, M.; Zhu, C.; Xu, J. Tear resistant Tyvek/Ag/poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate (PEDOT:PSS)/carbon nanotubes electrodes for flexible high-performance supercapacitors. Chem. Eng. J. 2021, 420, 127665.
- Li, J.; Yan, W.; Zhang, G.; Sun, R.; Ho, D. Natively stretchable micro-supercapacitors based on a PEDOT:PSS hydrogel. J. Mater. Chem. C 2021, 9, 1685–1692.
- Karade, S.S.; Sankapal, B.R. Room temperature PEDOT:PSS encapsulated MWCNTs thin film for electrochemical supercapacitor. J. Electroanal. Chem. 2016, 771, 80–86.
More
Information
Subjects:
Materials Science, Textiles
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
08 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




