Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Textiles
Flexible supercapacitors are highly demanding due to their wearability, washability, lightweight property and rollability. Supercapacitors are specially designed capacitors which have huge capacitance value and energy density when compared to the conventional capacitors that are with fast storage ability and high energy density than capacitors
- supercapacitor
- electrical conductivity
- energy storage
- banana peel
1. Introduction
Supercapacitors are specially designed capacitors which have huge capacitance value and energy density when compared to the conventional capacitors that are with fast storage ability and high energy density than capacitors [15]. Based on the working and energy storage principle, supercapacitors are categorized into three basic groups as shown in Figure 1. Electric double-layer capacitors (EDLCs) are types of capacitors constructed using three materials the so-called electrodes, electrolytes, and a separator. They are portable, very efficient, and high-power energy storage devices [16]. EDLCs store energy by means of non-faradic principle or electrostatically that encompasses no transmission of charge between the electrolyte and electrode.
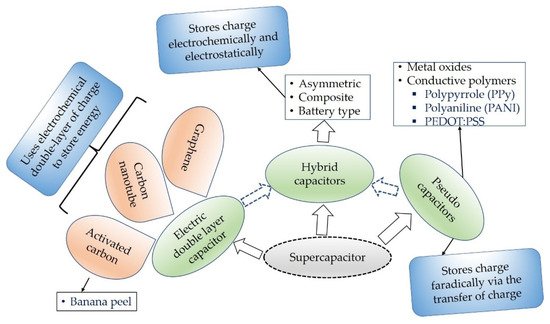
Figure 1. Types of supercapacitors based on its working principles.
Pseudocapacitors on the contrary to EDLCs store charge via the transfer of charge amongst the electrode and electrolyte faradically [17]. Pseudocapacitors employed various conductive polymers such as polypyrrole (PPy) and polyaniline (PANI) [17], poly(3,4-ethylenedioxtthiophne) polystyrene sulfonate (PEDOT:PSS) [18]. Due to the charging and discharging behavior and the reduction-oxidation rection occurred during processing, pseudocapacitors lack stability. Stability against the environment is very crucial for such types of materials.
Hybrid supercapacitors for energy storage principle is based on the combinations of the EDLCs and pseudocapacitors. Hybrid supercapacitors comprise the compensations of pseudocapacitors and EDLCs [19]. The limitations in both supercapacitor types are eliminated in the hybrid system and show better electrochemical characteristics.
2. Supercapacitors Based on Carbonized Banana Peels
Banana peels are conventionally waste materials that habitually discarded after consuming the edible parts. This causes a temporary pollution to the surrounding area where high marketing areas are available. On the other hand, the depletion of fossil fuels along with the dynamic climatic alteration requires extra exploitation of spotless and justifiable energy alternative sources [8]. In addition, the recent surge of flexile and lightweight electronics with high durability pushes most industries to the implementation of the best alternatives to renewable energy sources. Among the flexible and lightweight energy storage devices, supercapacitors are widely researched. Supercapacitors are the most promising energy storage alternatives for short-term applications. Supercapacitors have high power density and fast charge-discharge rate beyond they do have long lasting life cycle against the surrounding environment [20].
Carbon-based porous structure materials have been proven to be promising as flexible, lightweight, and durable supercapacitors [20]. Porous supercapacitors based on carbonized banana peel is one of the promising research areas in recent years [9,21,22,23]. Banana peel can be prepared for a possible supercapacitor in different ways. Figure 2 illustrates one route of manufacturing supercapacitor from carbonized banana peel.
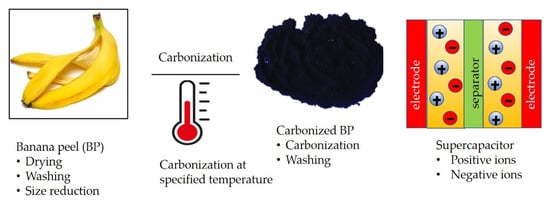
Figure 2. Illustrations showing process of manufacturing flexible supercapacitors starting from raw material preparation, carbonization and supercapacitor assembly.
Banana peel is an excellent potential for manufacturing supercapacitors. Various carbonization methods have been employed to improve the performance of supercapacitors with irrespective of its purposes and achieved high capacitance values. Table 1 summarizes the applied carbonization methods and its effect on the capacitance values.
Table 1. Summary of carbonized supercapacitor (SC) manufacturing based on banana peel.
| Method of Carbonization |
Purposes | Results Achieved | Ref. |
|---|---|---|---|
| One-step chemical activation | Improving electrochemical performance | Capacitance of 227 Fg−1 at 1 Ag−1 | [24] |
| Carbonization without activation | Green and cost-effective facile route | Notable specific capacitance (811 Fg−1) | [21] |
| one-step hydrothermal method | To get excellent electrochemical performance | Capacitance enduring 51 Fg−1 at 5.0 Ag−1 | [25] |
| Chemical activation | To improve conductivity and electrochemical performance | Specific capacitance of 90.23 Fg−1 at 10 mVs−1 |
[26] |
| Heating banana peel soaked with KOH at high temperature | To check stability against multiple electronic cycling and bending | Devices displayed high areal capacitance of 88 mF/cm2 at 10 mV/s scan rate | [27] |
| two-step hydrothermal process | To reach easily to the active site and to shorten the ion transport path | Large capacitance of 816 Fg−1 at the current density of 5 mA cm−2 | [28] |
| Green pyrolysis | To check energy storage ability and environmental remediation | Capacitance of 655 Fg−1 in 1 M at a current density of 0.35 Ag−1 & excellent cyclic stability of 79.3% | [29] |
| Sulfur-doped (chemical carbonization) | Sustainable supercapacitor production | High Brunauer-Emmett-Teller surface area of 2224.9 m2/g, a large pore volume of 0.77 cm3/g | [22] |
| Carbonization with chemical activation | To see relationship of surface area to cell capacitance | SC increased from 59–~265 Fg−1 at 0.1 Ag−1 | [30] |
| Biological activation | Optimization of precursors and synthesis methods | Get specific capacitance of 476 Fg−1 in 1 M H2SO4 electrolyte | [31] |
| Chemical co-precipitation method | To get high electrochemical property | specific capacitance of 465 Fg−1 at a scan rate of 10 mV s−1 by CV | [23] |
| Biological fermentation | Stabilize the structure of electrodes | High-capacity hold of 58.35% after 100 cycles | [32] |
| Hydrothermal method | To increase the electrochemical performance | Had a specific capacitance of 139.6 Fg−1 at 300 mA g−1 | [33] |
| straightforward carbonization | To improve electrochemical performance | Specific capacitance improved from 59 to 258–273 Fg−1 at 0.1 Ag−1 | [34] |
| KOH pellets at different carbonization temperatures | To improve electrochemical performance | Specific capacitance of 165 Fg−1 at energy density of 18.6 Wh kg−1 at 0.5 Ag−1 | [35] |
| Carbonization followed by activation | To see the relationship between surface area and electrochemical property | Surface area had significant effect on electrochemical property (specific capacitance of 68 Fg−1) | [36] |
| H2SO4 activation | Carbon nanofiber synthesis | Carbon nanofiber formed at 700 °C) | [37] |
| KOH | Absorption study | reflection loss peak of −44.59 dB at 10.84 GHz | [38] |
As indicated in Table 1, carbonization of biomass resulted in carbon materials with porous structure and hence carbon materials have used for energy storage materials as supercapacitors [39]. Recent developments in the production of flexible supercapacitors indicated that banana peels represent a possible method to produce durable, flexible, and light weight energy storage devices. Supercapacitors based on banana peel achieved high capacitance value due to the ability of embodying high surface area which in turn can encompass highly porous structures. This most likely proves that carbon materials based on banana peels will continue to strive to be one of the protentional applications for supercapacitor production as far as optimization in terms of activation type, high porosity, and flexibility achieved.
Banana peels (BP) have been used as the supercapacitor electrode after carbonization. They can be carbonized by applying various mechanisms such as chemical activation [40], hydrothermal methods [33], biological fermentation [32], and pyrolysis [29]. For the sake of obtaining higher capacitance value, various chemicals may be employed to chemically activate the banana peel carbon. The chemical activation may increase the surface area of the carbonized banana peel with affecting the structure of activated carbon. Activation helps to increases the surface area to volume ratio and to enhance absorption is enhanced. Moreover, the supercapacitor for chemically-activated banana peel achieved exceptional recurring steadiness with capacitance maintenance of ~97% for 5000 cycles [24]. Banana peel carbon provides the highest performance when activated using chemicals. Furthermore, the chemical precipitation has helped to enhance the supercapactive performance of carbonized supercapacitors, and hence chemical activation in suggested for the production of durable supercapacitors in the future.
3. Supercapacitors Based on PEDOT:PSS
3.1. Introduction
Poly(3,4-ethylenedioxythiophne)-polystyrene sulfonate (PEDOT:PSS) is the most recently explored, widely used, and successfully implemented intrinsically conductive polymers. The utmost plausible reason for this is due to excellent electromechanical performance [41], high conductivity and wash durability [42], and water processability and light transmissive behaviour [43,44]. In addition to this point, PEDOT:PSS is stable in environment and can be processed up to high temperature ranges. PEDOT:PSS alone is not good conductor as hydrophilic PSS hides the conductivity. In this case, some researchers have been using polyethylene glycol (PEG) [35], dimethyl sulfoxide (DMSO) [36], and ethylene glycol (EG) [37] to enhance the conductivity. When PEDOT:PSS is doped with these solutions, structural rearrangement has taken place (Figure 3c) [42].
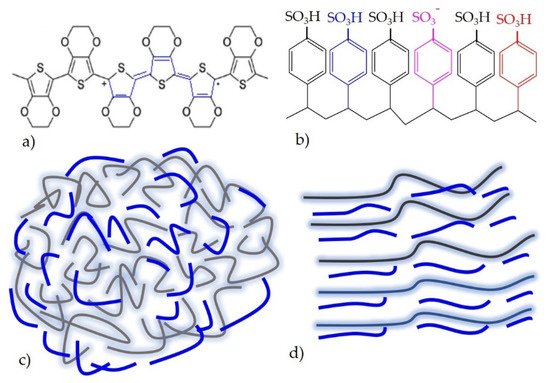
Figure 3. (a) Chemical structure of PEDOT; (b) chemical structure of PSS; (c) when PSS is added to PEDOT; a water dispersion solution is created; (d) when PEDOT:PSS is doped with conductive enhancers, a structural re-arrangement is formed, adapted from Ref. [42].
Perhaps the most well-known example of an organic intrinsic semiconductor polymer is the polymer poly(3,4-ethylenedioxythiophene) (PEDOT), predominantly when it intricates with poly(styrene sulfonate-PSS) (PEDOT:PSS). PSS surrounds and mostly found on the surface of PEDOT and which helps PEDOT to be dispersible in water. It is extremely conductive, light-transmissive to a significant extent, water-processable, and highly flexible. Considerable recent research on this ubiquitous material has focused on enhancing its deformability beyond flexibility (a property that any suitably thin material possesses) to stretchability (a property that requires molecular or nanoscale structure engineering). The aforementioned properties of PEDOT:PSS are fundamental, which may help researchers to investigate the use of PEDOT:PSS as a durable energy storage materials such as supercapacitors.
Poly(3,4-ethylenedioxythiphene)-poly(styrene sulfonate) has been used in the production of high-performance supercapacitors. Table 2 summarizes the use of PEDOT:PSS to produce supercapacitor and its efficiency. Using PEDOT:PSS conductive polymer not only brought electrochemical performance but also possess excellent durability against washing [41].
Table 2. Summary of supercapacitors based on PEDOT:PSS with capacitance (C) main factor.
| Precursor/Composite | Capacitance | Performance | Ref. |
|---|---|---|---|
| rGO | 226.5 F cm−3/279.3 mF cm−2 at 0.5 A cm−3 | 74.7% C retention at 50 A cm−3 | [45] |
| WO3 | 1139.6 mF cm−2 at 2 mA cm−2 | Working voltage of 1.6 V | [46] |
| Cellulose nanofibrils | 854.4 mF cm−2 at 5 mV/s | Areal ED of 30.86 μWh cm−2 | [47] |
| PANI, PPy | 156 mF cm−2 C at 1 mA cm−2 CD | 41% capacity persisted at 20 mA cm−2 | [48] |
| MgTf2 | 280 Fg−1 at 3 mV/s and 376.6 Fg−1 at 100 mA g−1 | PD ~100.08 Wkg−1 | [49] |
| Graphene | C of 2 mF cm−2 at a scan rate of 102 mV s−1 | >95% C retaining after 103 cycles | [50] |
| Polypyrrole | 12.4–10.5 F cm−3 at a CD of 40–320 mA cm−3 | C retention rate of 88.1% for 103 charges/discharge cycles | [51] |
| Carbon nanofibers | C of 1321 Fg−1, at a scanning speed of 1 mV/s | Retention of 80% of its performance after 2500 CV cycles | [52] |
| MnO2 microspheres | Capacitance of 135.4 mF cm−2 | 94% C maintenance after 3000 cycles | [53] |
| rGO/CoFe2O4 | Capacitance of 229.6 mF cm−2 | ED and PD of 25.9 Wh kg−1 and 135.3 W kg−1, respectively | [54] |
| Poly(acrylamide) | specific C of 327 Fg−1 at 3 mV/s | highest ionic conductivity of 13.7 × 10−3 S/cm at 22 ± 2 °C | [55] |
| CoCCHH-CoSe | C of 440.6 Fg−1 at 1 Ag−1 | ED of 137.7 Wh kg−1 | [56] |
| PANI Nanofiber | C of 301.71 mF cm−2 at CD of 1 mA cm−2 | ED of 0.023 mWh cm−2, with PD of 0.279 mW cm−2 at a lower current density of 1 mA cm−2 | [57] |
| nanoflower MnOx | C of 580 mF·cm−2 at 0.5 mA | >90% for 40% stretch | [58] |
| ---- | C of 3.92 mF/cm2 at 1 mA/cm2 | C retention > 90% after 3 × 103 cycles | [59] |
| ---- | Capacitance of 990 mF cm−2 | C retention of 74.7% after 14,000 cycles | [60] |
| Alginate/PPy | Capacitance of 246.4 mF cm−2 | 97% of initial values after 180° bending | [61] |
| Ag-coated Tyvek | Mass C (138.7 Fg−1) & volume C (544.2 F/cm3) at the scan rate of 50 mV/s. | 91.2% retention after 100 cycles | [62] |
| PVA/H2SO4 | Areal C of 44.5 mF cm−2 at PD of 0.04 mW cm−2 | 92% retention at 200% stretchability | [63] |
| MWCNT | specific capacitance of 235 Fg−1 at 5 mV s−1 | retention of about 92% in 1M H2SO4 electrolyte | [64] |
GO, reduced graphene oxide; WO3, tungsten trioxide; MgTf2, magnesium trifluoromethanesulfonate; CD, current density; PD, power density; ED, energy density; CoCCHH-CoSe, heterogenous tube; MWCNT, multi-walled carbon nanotubes.
This entry is adapted from the peer-reviewed paper 10.3390/en15072471
This entry is offline, you can click here to edit this entry!
