
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Do Sung Huh | -- | 4401 | 2022-04-04 08:34:42 | | | |
| 2 | Do Sung Huh | -86 word(s) | 4315 | 2022-04-05 03:12:49 | | | | |
| 3 | Do Sung Huh | Meta information modification | 4315 | 2022-04-05 03:16:06 | | | | |
| 4 | Bruce Ren | -6 word(s) | 4309 | 2022-04-06 03:24:05 | | | | |
| 5 | Bruce Ren | + 1 word(s) | 4310 | 2022-04-06 10:24:05 | | |
Video Upload Options
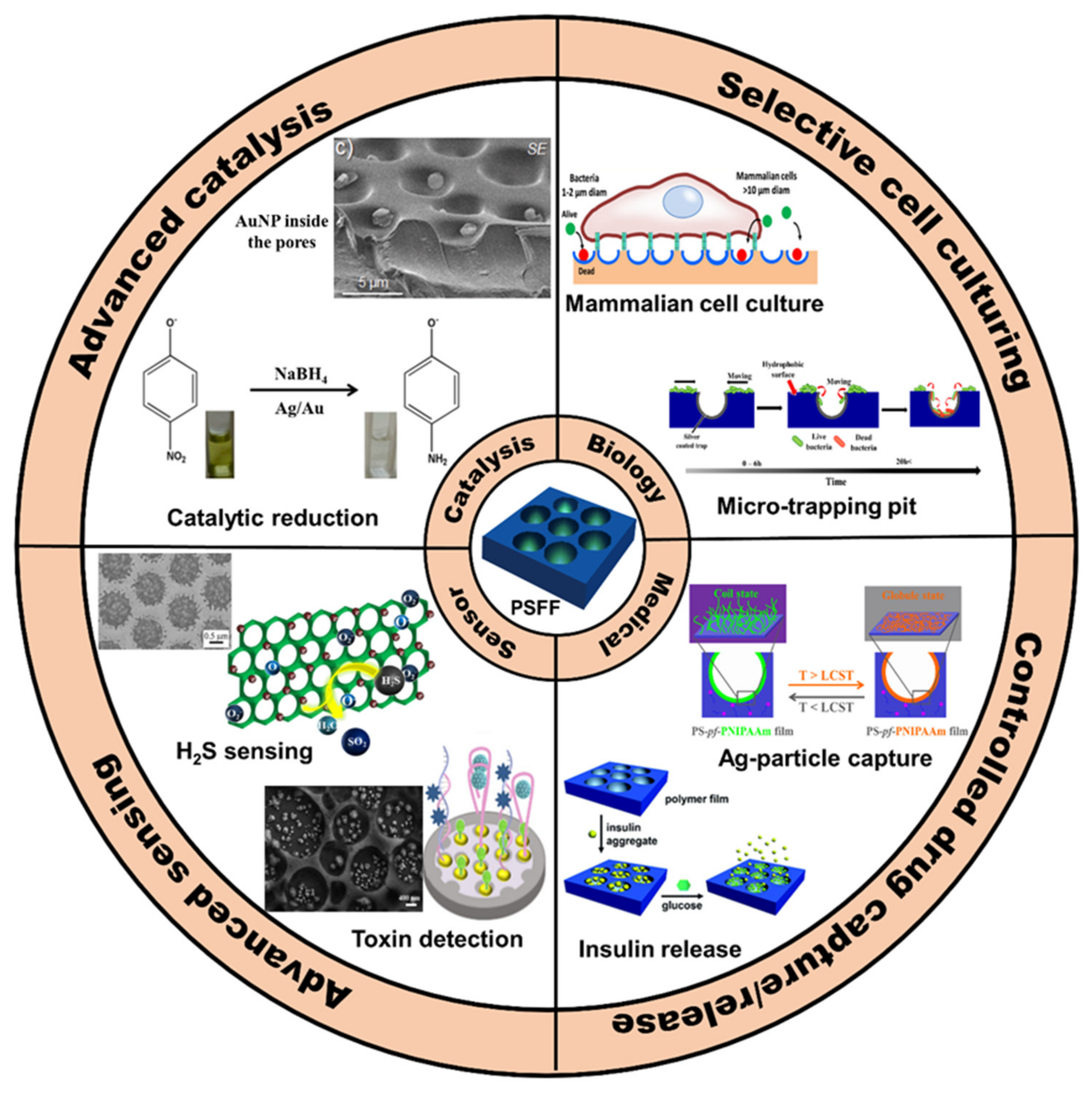
Recent developments in the field of the breath figure (BF) method leading to pore-selective functionalization of honeycomb-patterned (HCP) films attracted great interest. The pore-selective functionalization of the HCP film gives unique properties to the film which can be used for specific applications like protein recognition, catalysis, selective cell culturing, and drug delivery.
1. Introduction
The functionality exclusive to the surface of the pores enables the use of these arrays in technical applications, such as the site-specific immobilization of proteins, essential to avoid hampering the activity of the protein which is beneficial to the specific selective adsorption of protein [1][2]. The protein orientation plays a critical role if protein microarrays are to be used as quantitative tools in biomedical research or clinically important protein-based nanomedicines [3]. For example, the surfaces prepared could be of potential interest as 3D cell culture platforms, that have been otherwise obtained via tedious multi-step procedures using lithographic techniques. The cell adhesion and proliferation on HCP films can be controlled by polymer film surface chemistry, which can be directed into the pores by functionalizing the pore-interior with glycopolymers or proteins [4][5][6]. This can enhance their proliferation and retain spherical morphology. Moreover, the specific patterning of metal or semiconductor materials could be applied in photonics, since the photonic band structure depends on the spatial periodic structures and the refractive indices of the dielectric materials in the structure [7].
2. Pore-Selective Functionalization of HCP Films by Self-Assembly
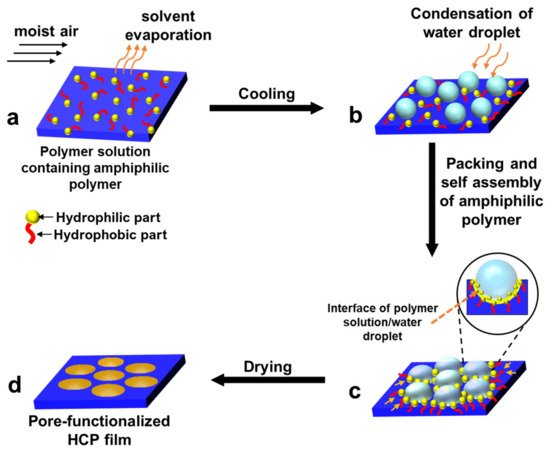
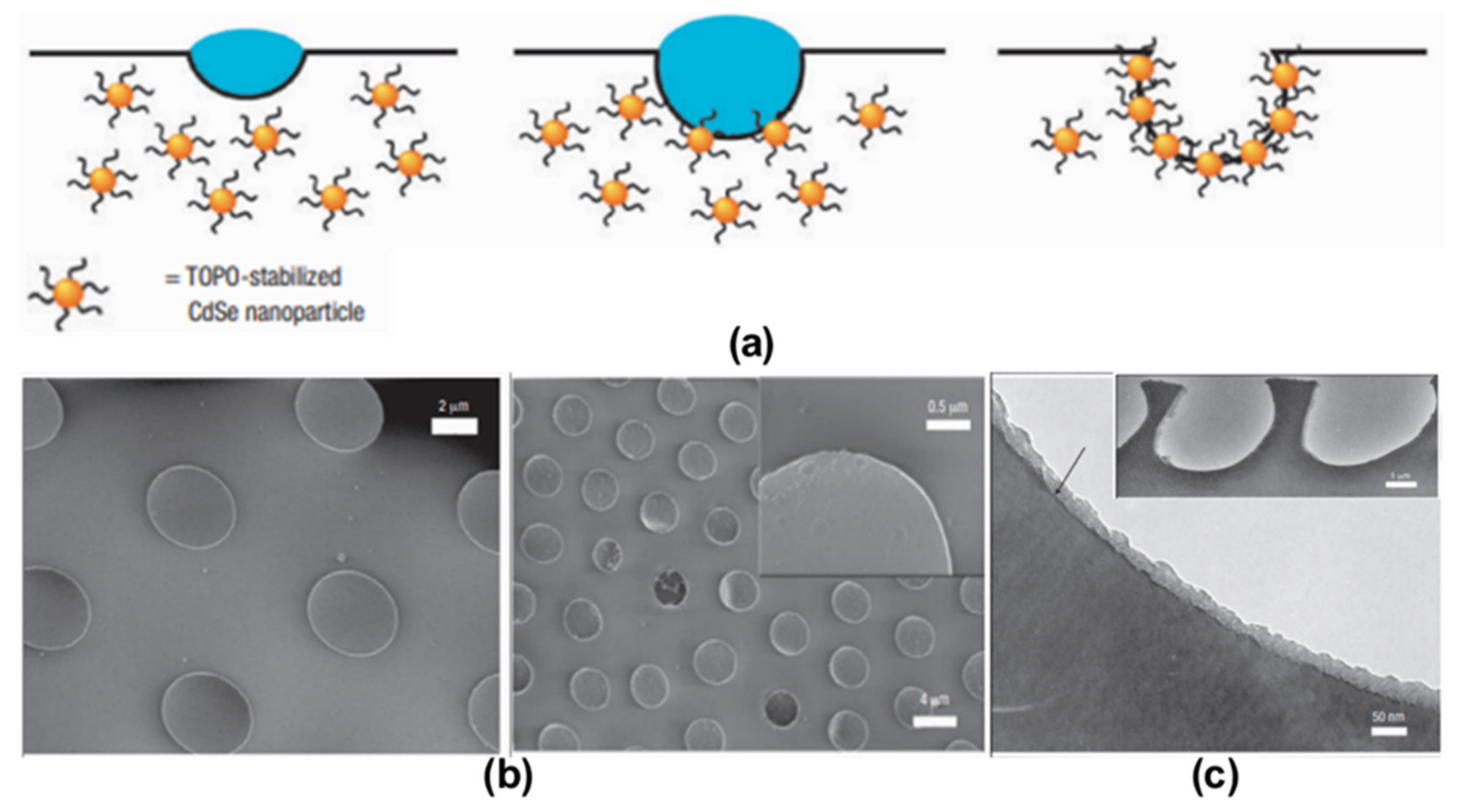
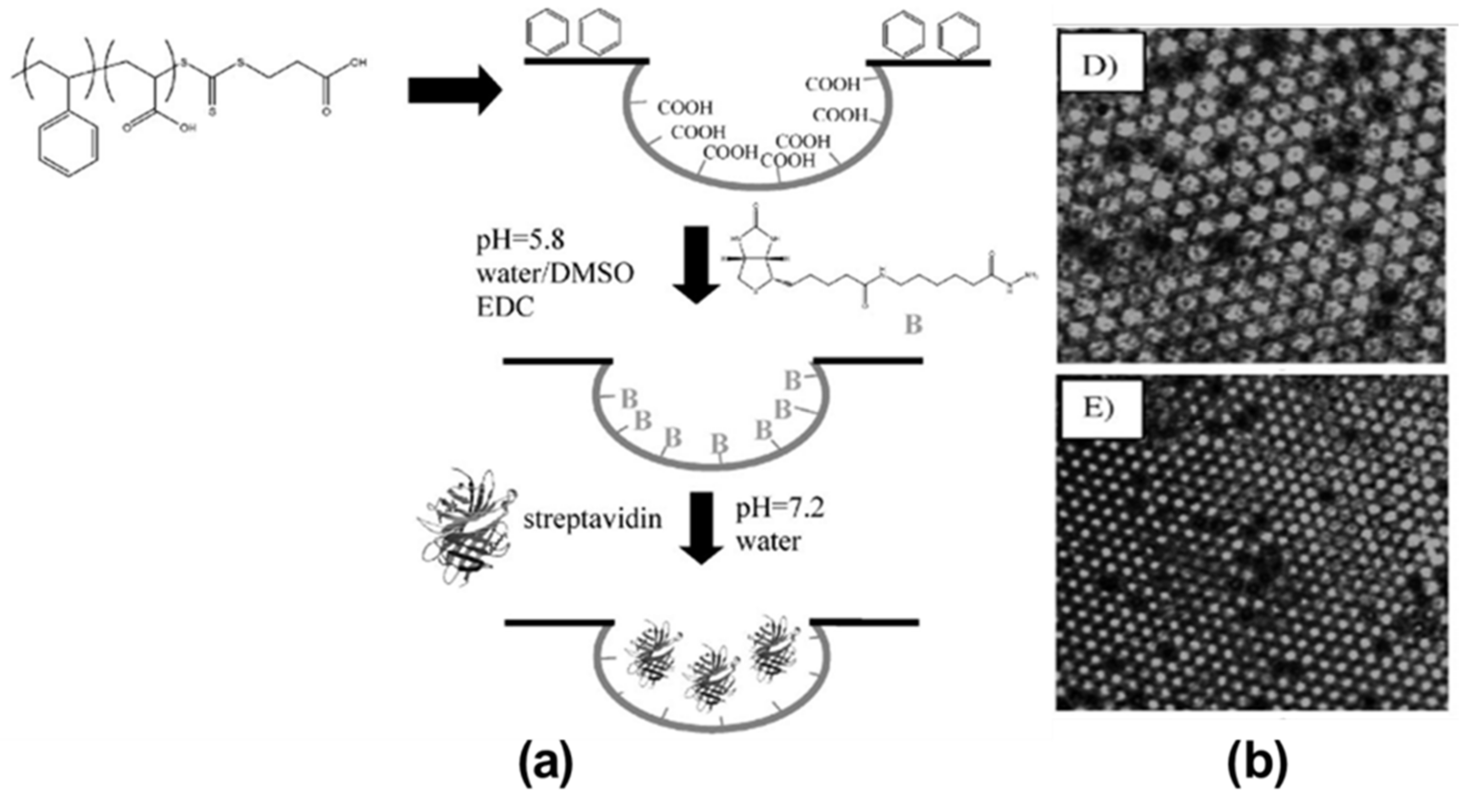
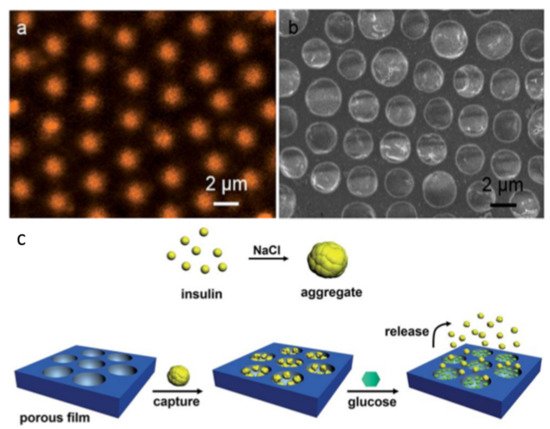
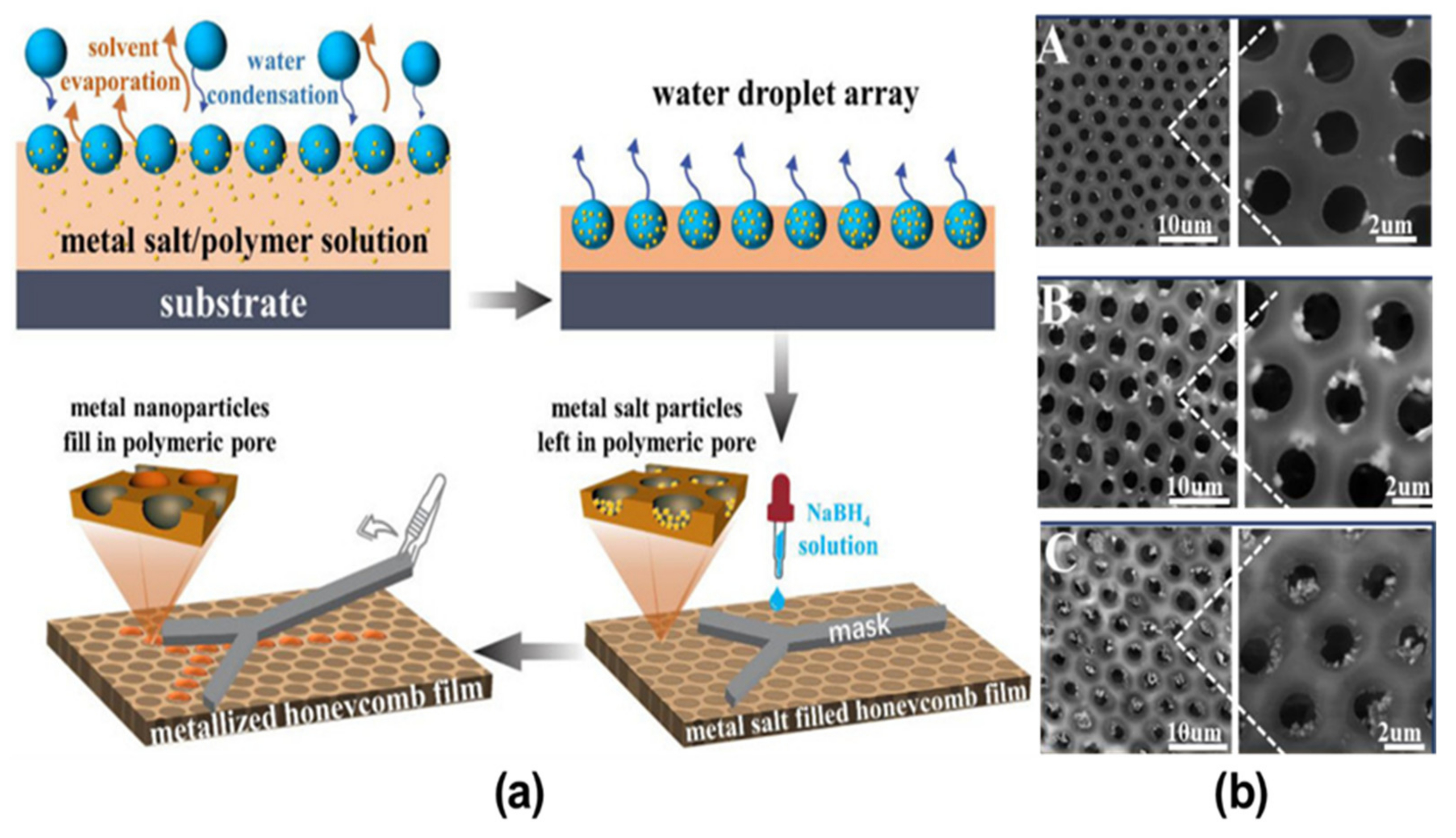
3. Pore-Functionalization by Self-Assembly Followed by Additional Treatment
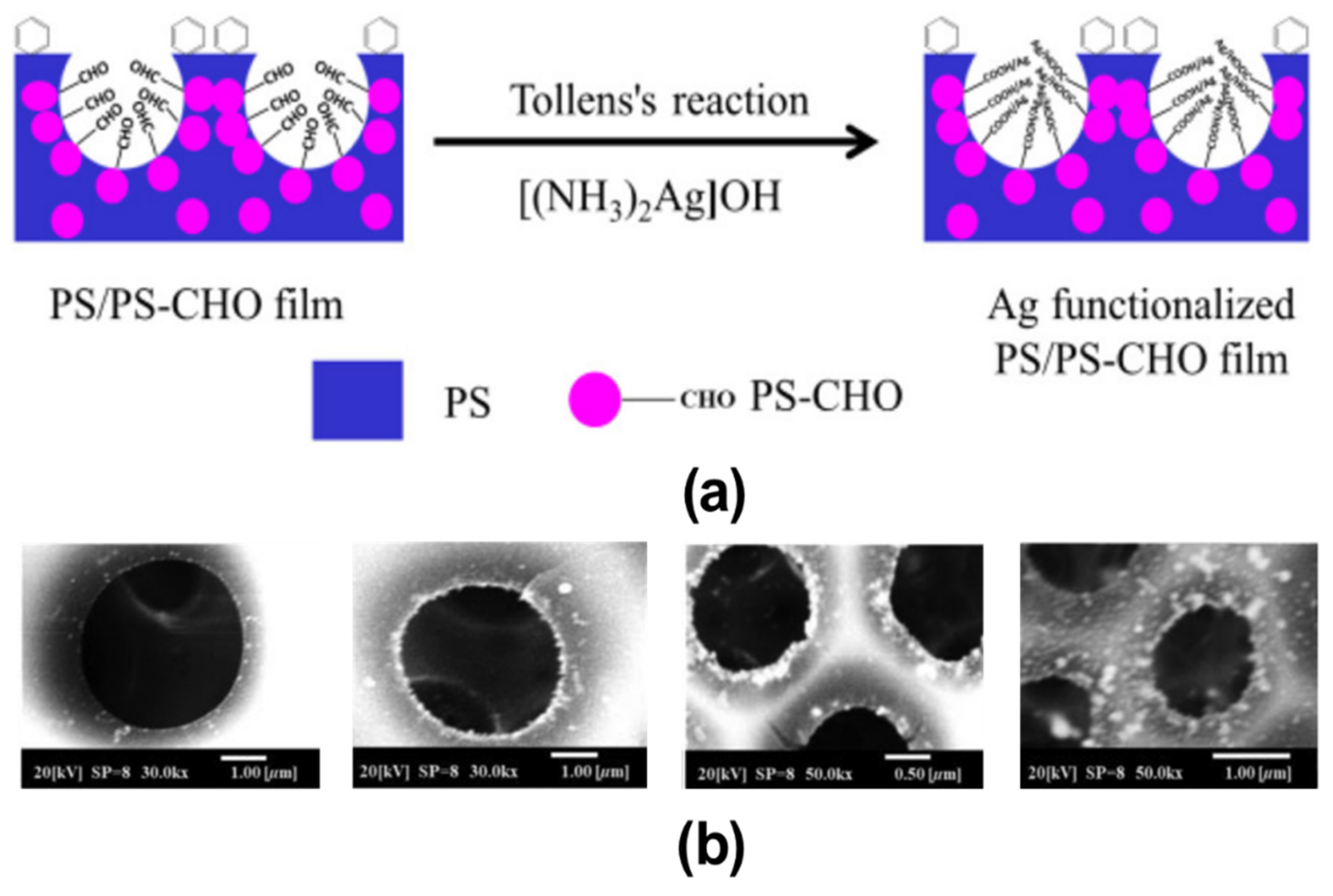
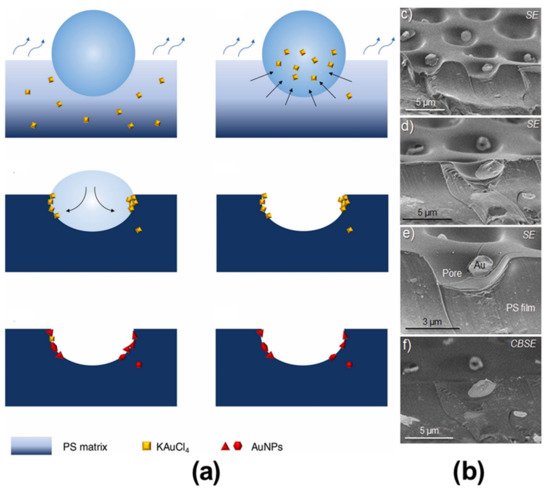
3. Pore-Selective Functionalization of HCP Films Accompanying Chemical Reaction
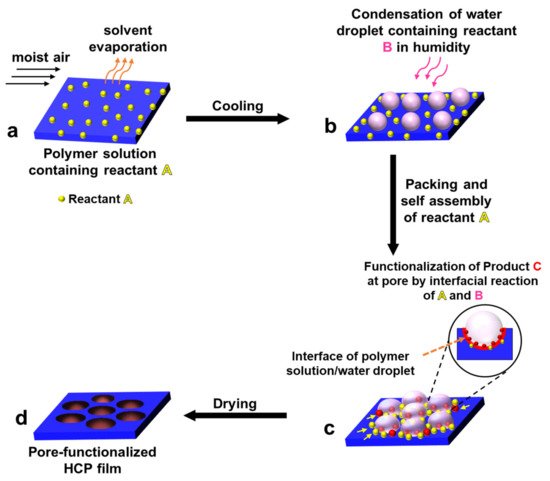
3.1. Pore-Functionalization by BF Process Using Water as a Reactant
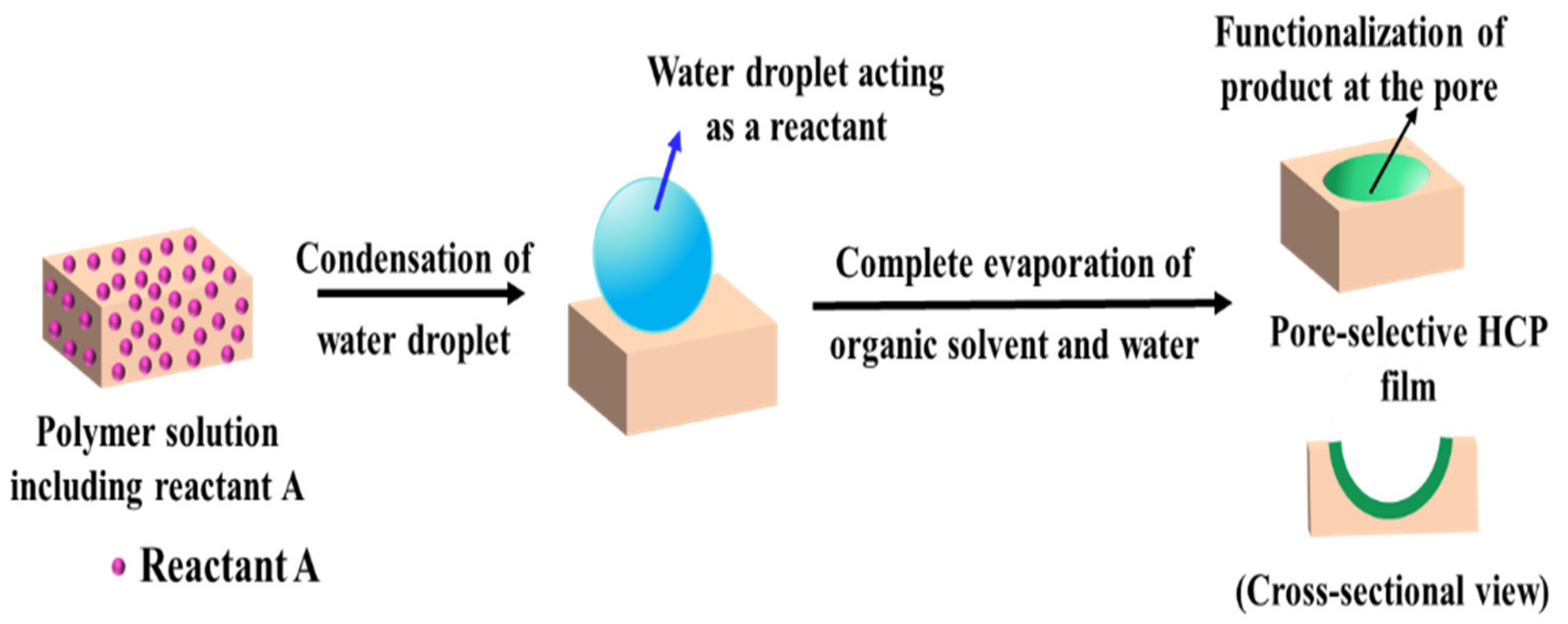
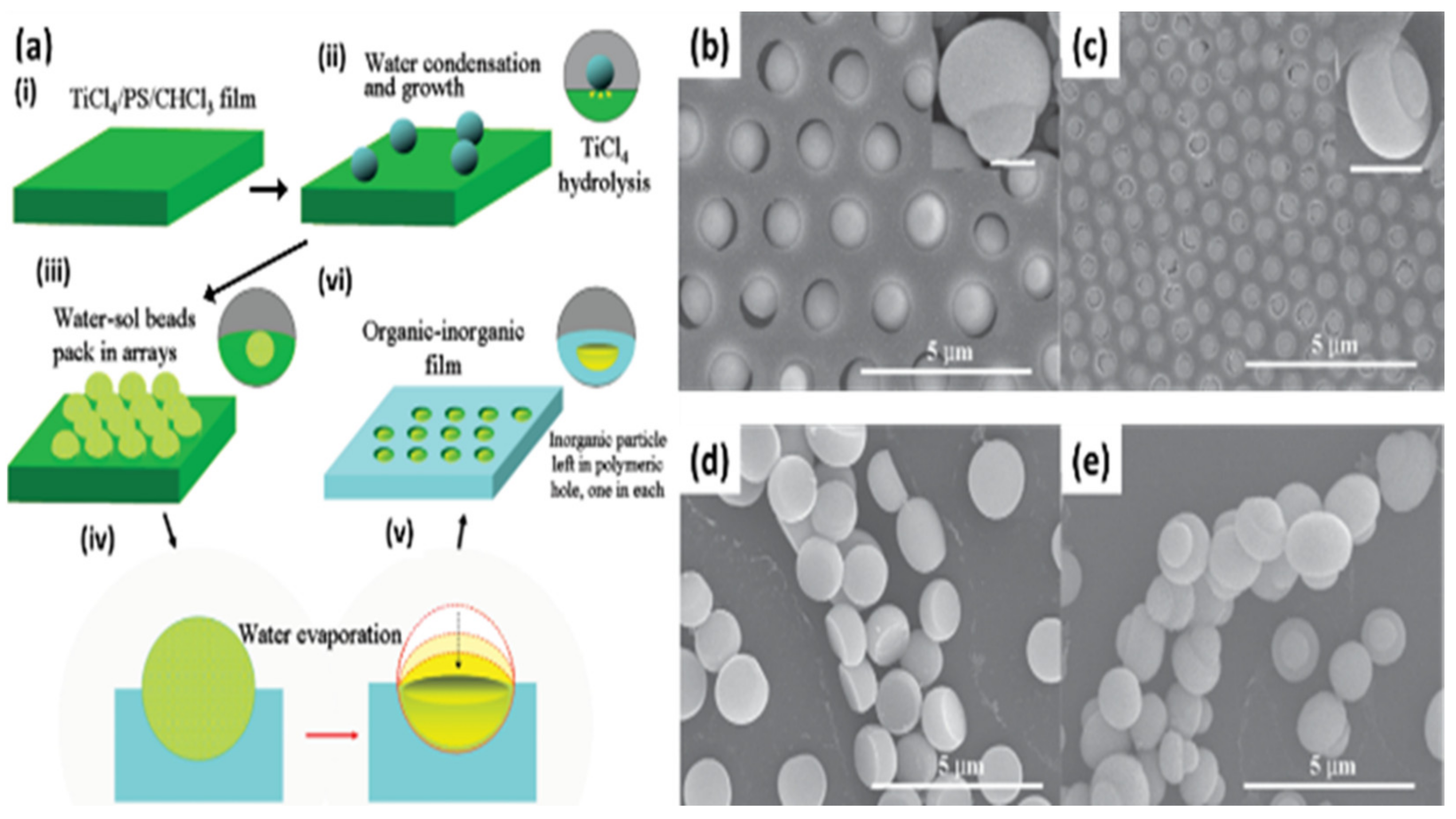
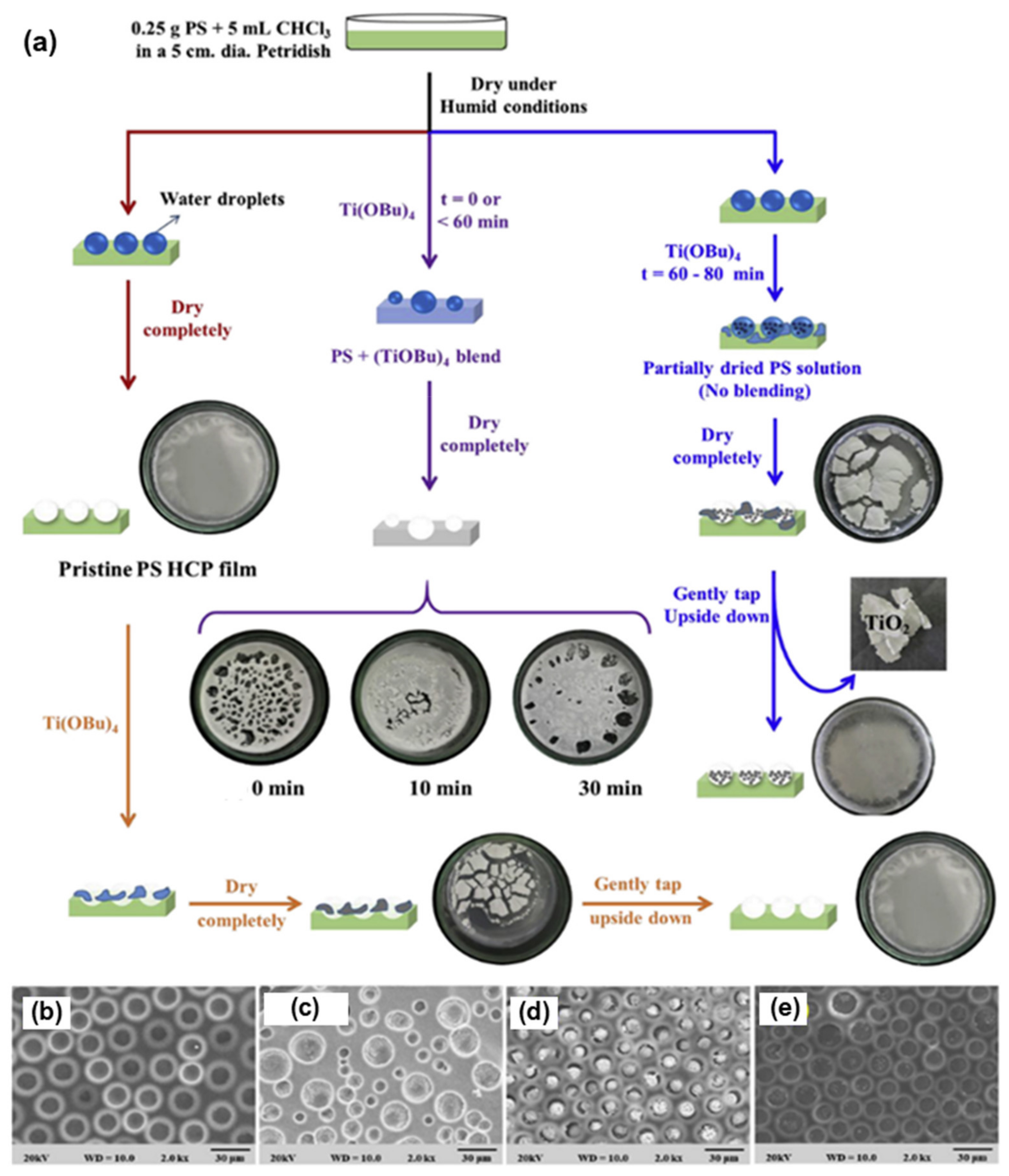
| Reactant A in the Polymer Solution | Humid Conditions | Product/Functionalization in Pore | Ref. |
|---|---|---|---|
| Titanium tetrachloride | Water | Titanium dioxide | [101] |
| Titanium tetrachloride | Water | Titanium dioxide | [102] |
| Tin tetrachloride | Water | Tin dioxide | [103] |
| Titanium butoxide | Water | Titanium dioxide NPs | [104] |
| Titanium n-butoxide | Water | Titanium dioxide | [107] |
| Titanium tetraisopropoxide | Water | Titanium dioxide | [108] |
| Alkylcyanoacrylate | Water | PACA | [106] |
2.3.2. BF Process Accompanying Chemical Reactions with a Non-Aqueous Reactant
| Reactant A in the Polymer Solution | Reactant B in Humid Conditions | Functionalization in Pore (Product C) |
Ref. |
|---|---|---|---|
| Poly (4-vinylpyridine) | Formic acid | PVP-FA precipitate | [120] |
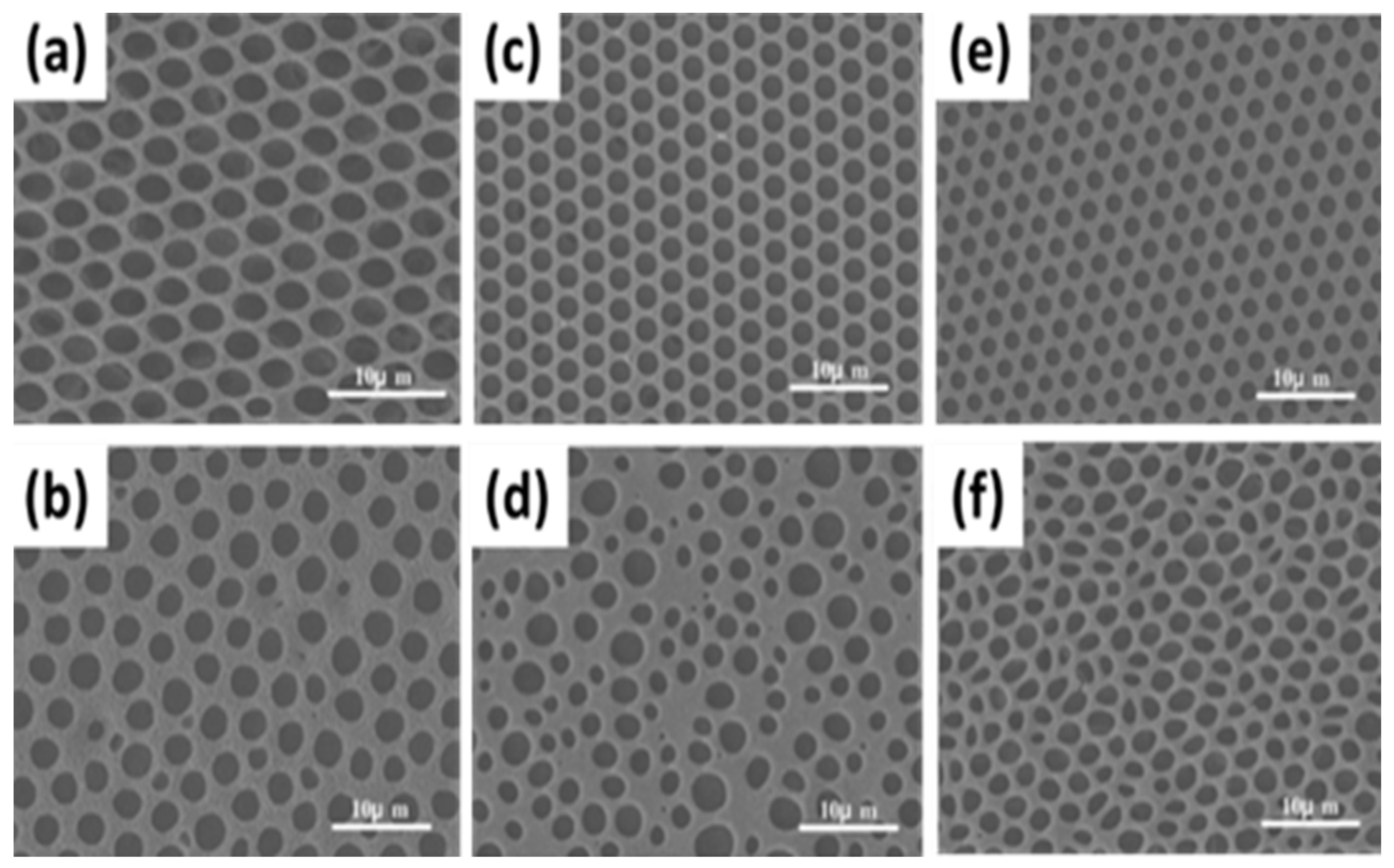
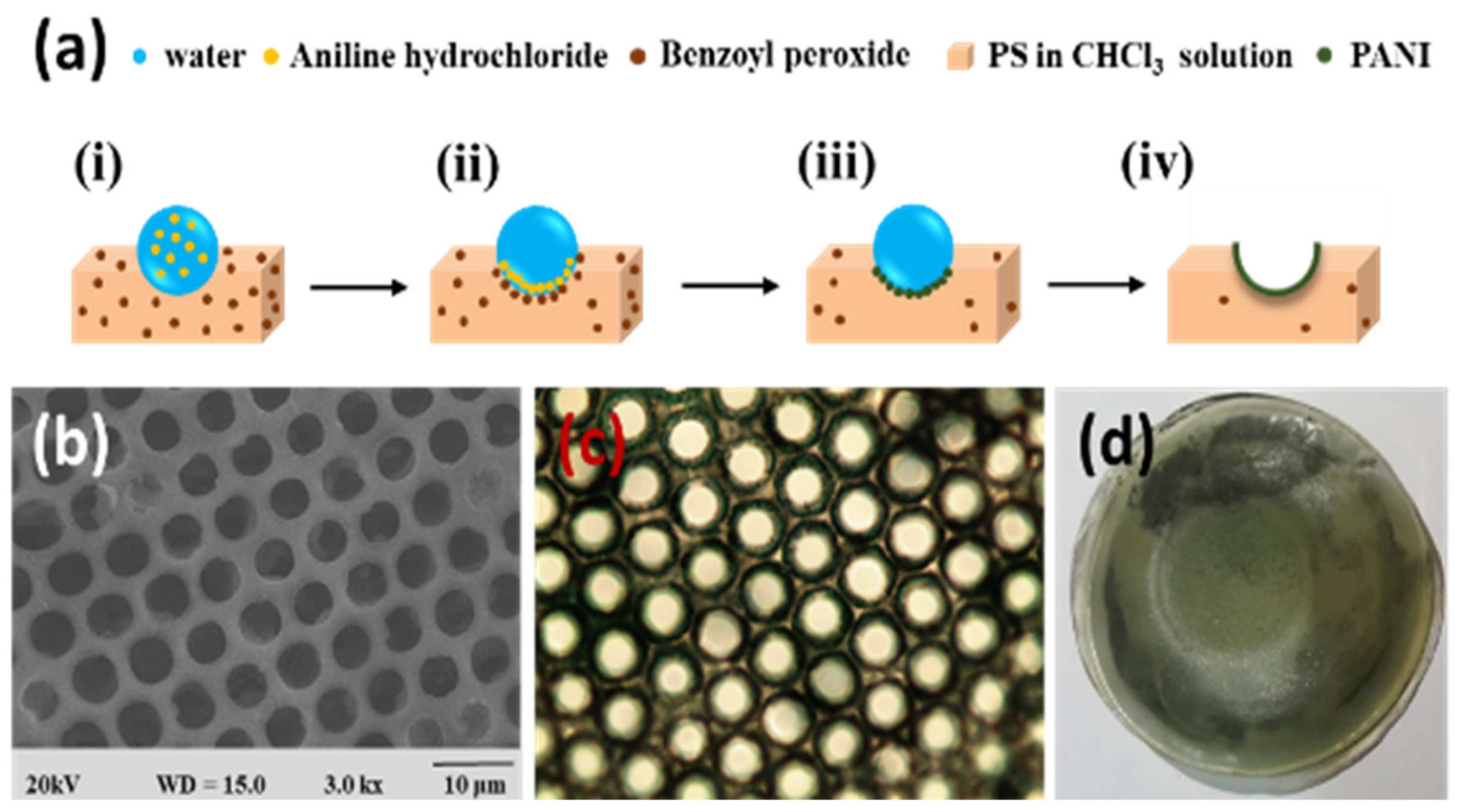
| Benzoyl peroxide | Aniline | Polyaniline | [110] |
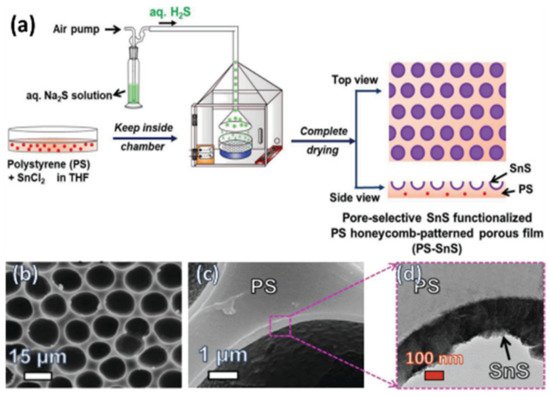
| Tin dichloride | Hydrogen sulfide | Tin sulfide | [109] |
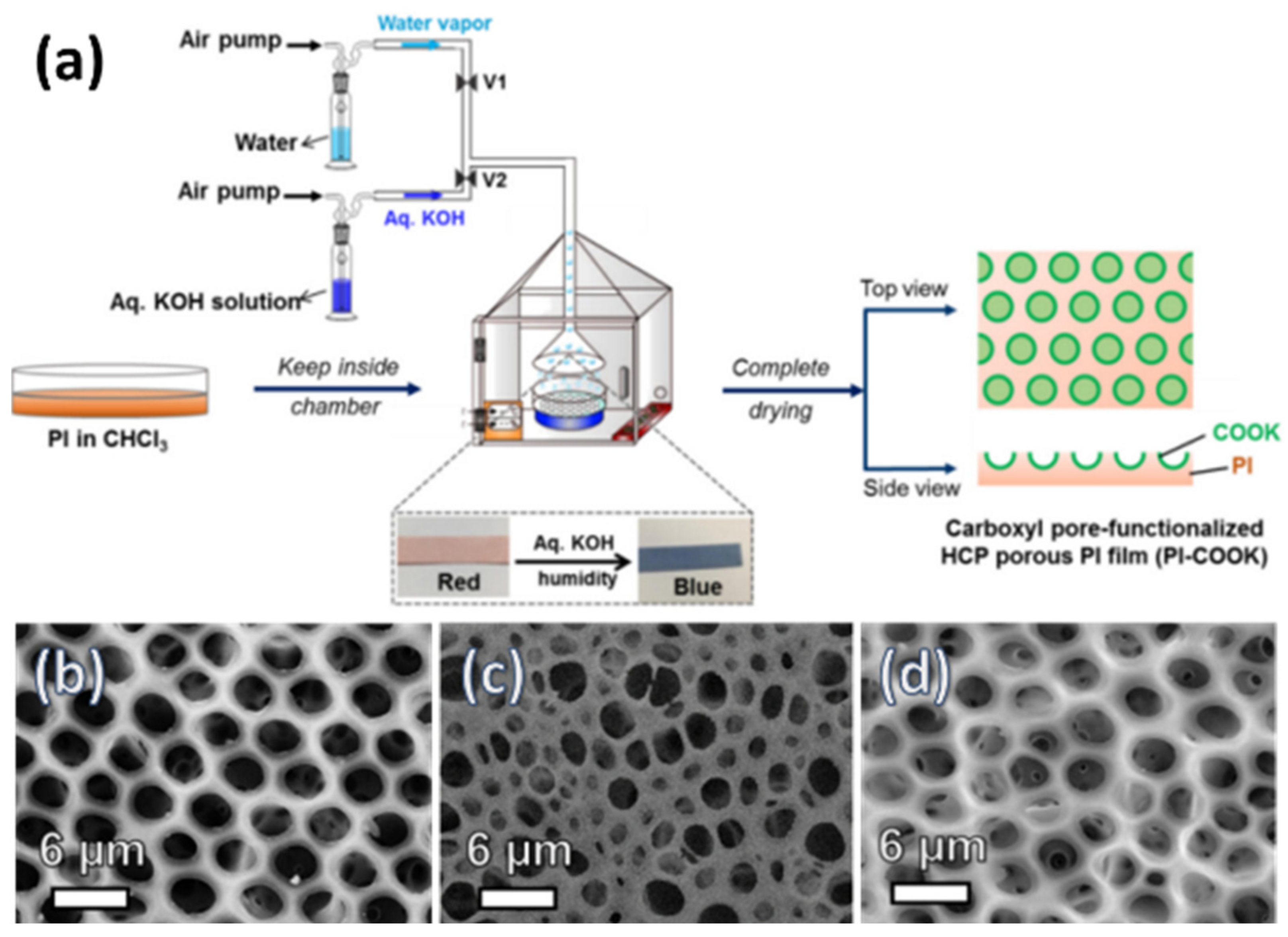
| Polyimide | Potassium hydroxide | Carboxylic group | [111] |
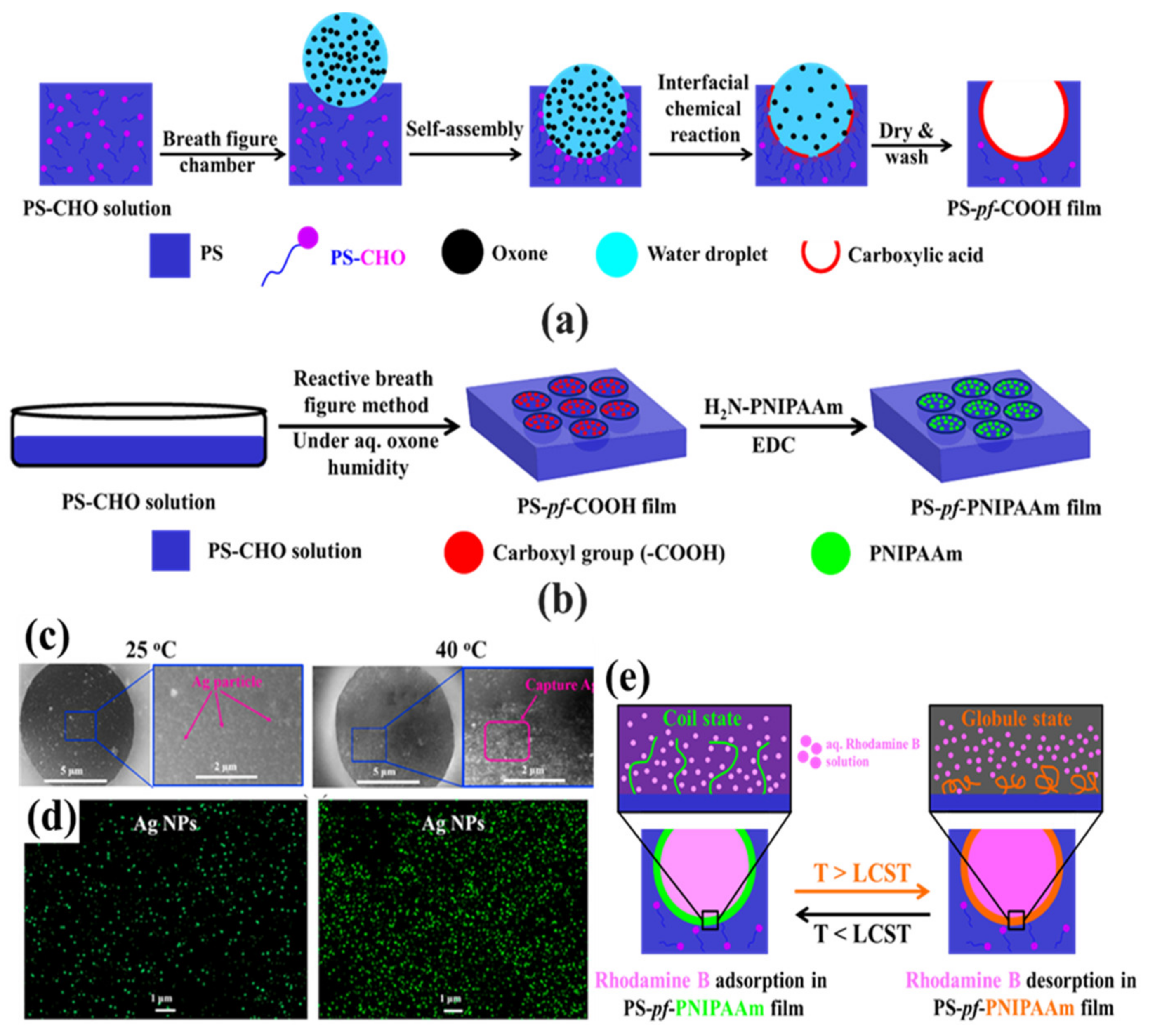
| PS aldehyde (PS-CHO) | Oxone | Carboxylic acid (–COOH) | [112] |
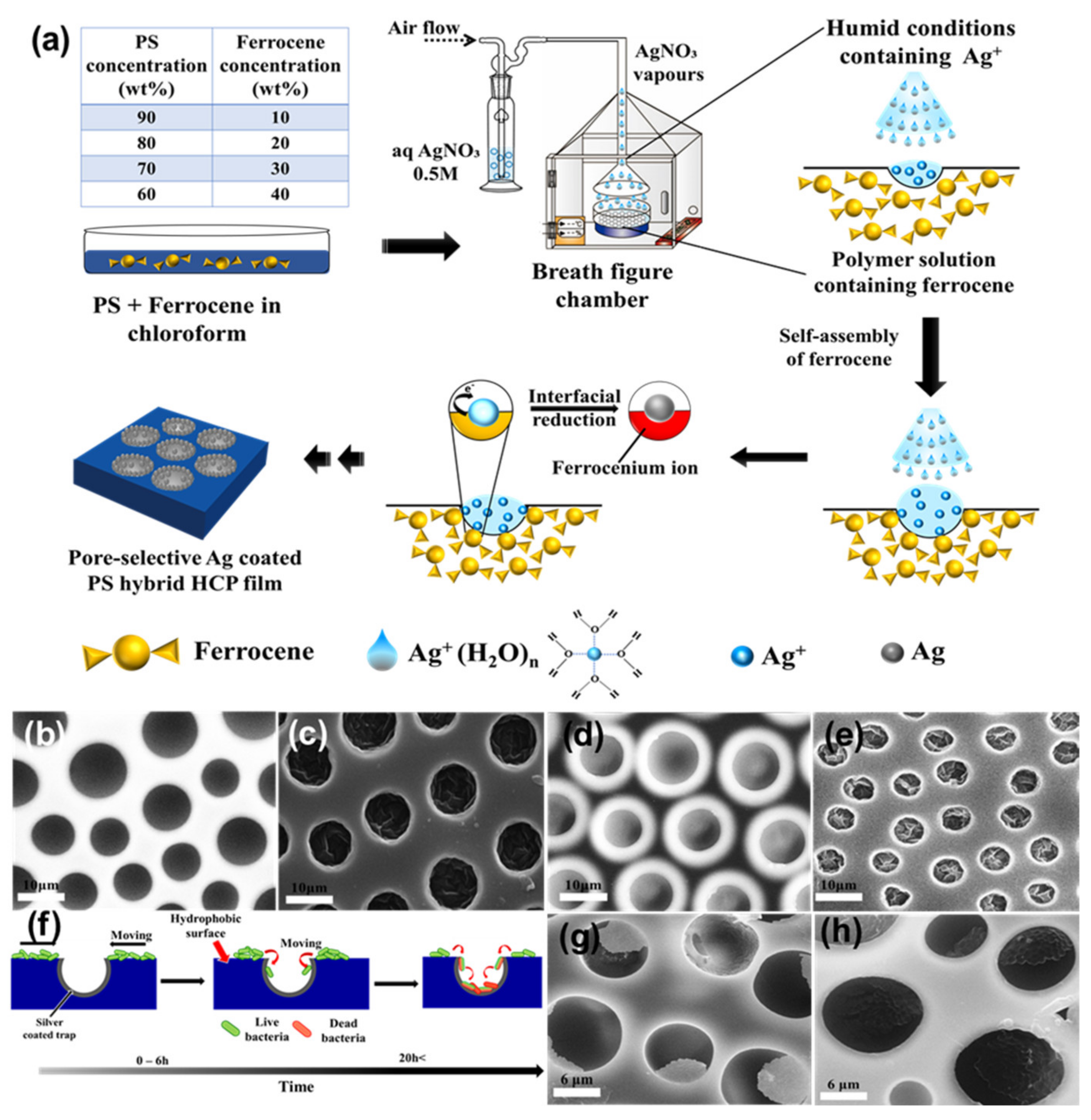
| Ferrocene | Silver Nitrate | Silver | [119] |
3. Conclusions
References
- Zhang, Y.; Wang, C. Micropatterning of Proteins on 3D Porous Polymer Film Fabricated by Using the Breath-Figure Method. Adv. Mater. 2007, 19, 913–916.
- Ke, B.-B.; Wan, L.-S.; Xu, Z.-K. Controllable Construction of Carbohydrate Microarrays by Site-Directed Grafting on Self-Organized Porous Films. Langmuir 2010, 26, 8946–8952.
- Cha, T.; Guo, A.; Zhu, X.Y. Enzymatic activity on a chip: The critical role of protein orientation. Proteomics 2005, 5, 416–419.
- Ke, B.-B.; Wan, L.-S.; Zhang, W.-X.; Xu, Z.-K. Controlled synthesis of linear and comb-like glycopolymers for preparation of honeycomb-patterned films. Polymer 2010, 51, 2168–2176.
- Muñoz-Bonilla, A.; Ibarboure, E.; Bordegé, V.; Fernández-García, M.; Rodríguez-Hernández, J. Fabrication of Honeycomb-Structured Porous Surfaces Decorated with Glycopolymers. Langmuir 2010, 26, 8552–8558.
- Rodríguez-Hernández, J.; Muñoz-Bonilla, A.; Ibarboure, E.; Bordegé, V.; Fernández-García, M. Honeycomb structured porous interfaces as templates for protein adhesion. J. Phys. Conf. Ser. 2010, 252, 012002.
- Ma, H.; Cui, J.; Chen, J.; Hao, J. Self-Organized Polymer Nanocomposite Inverse Opal Films with Combined Optical Properties. Chem. Eur. J. 2011, 17, 655–660.
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868.
- Yabu, H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822.
- Bormashenko, E. Breath-figure self-assembly, a versatile method of manufacturing membranes and porous structures: Physical, chemical and technological aspects. Membranes 2017, 7, 45.
- Wan, L.-S.; Zhu, L.-W.; Ou, Y.; Xu, Z.-K. Multiple interfaces in self-assembled breath figures. ChemComm 2014, 50, 4024–4039.
- Yuan, H.; Yu, B.; Cong, H.; Chi, M.; Cheng, Y.; Lv, C. Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method. Materials 2018, 11, 481.
- Deng, Z.; Wang, L.; Yu, H. Fabrication of honeycomb-patterned film using hyperbranched polyethylene-based copolymer. Eur. Polym. J. 2017, 93, 428–435.
- Yeh, S.-C.; Wu, C.-H.; Huang, Y.-C.; Lee, J.-Y.; Jeng, R.-J. In Search of a Green Process: Polymeric Films with Ordered Arrays via a Water Droplet Technique. Polymers 2019, 11, 1473.
- Böker, A.; Lin, Y.; Chiapperini, K.; Horowitz, R.; Thompson, M.; Carreon, V.; Xu, T.; Abetz, C.; Skaff, H.; Dinsmore, A. Hierarchical nanoparticle assemblies formed by decorating breath figures. Nat. Mater. 2004, 3, 302–306.
- Min, E.; Wong, K.H.; Stenzel, M.H. Microwells with patterned proteins by a self-assembly process using honeycomb-structured porous films. Adv. Mater. 2008, 20, 3550–3556.
- Liang, J.; Ma, Y.; Sims, S.; Wu, L. A patterned porous polymer film for localized capture of insulin and glucose-responsive release. J. Mater. Chem. B 2015, 3, 1281–1288.
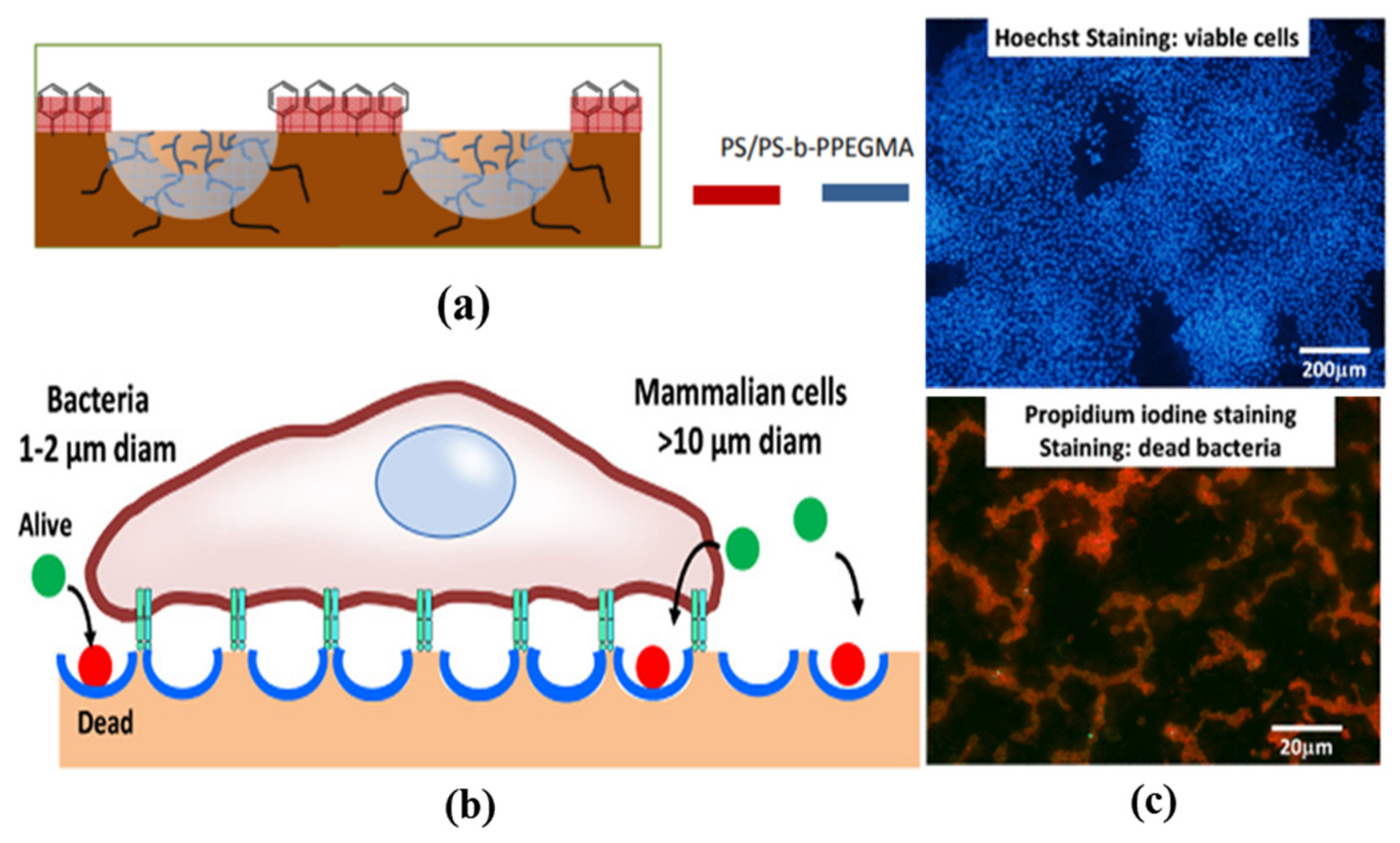
- Vargas-Alfredo, N.; Santos-Coquillat, A.; Martínez-Campos, E.; Dorronsoro, A.; Cortajarena, A.L.; del Campo, A.; Rodríguez-Hernández, J. Highly Efficient Antibacterial Surfaces Based on Bacterial/Cell Size Selective Microporous Supports. ACS Appl. Mater. Interfaces 2017, 9, 44270–44280.
- Martínez-Campos, E.; Elzein, T.; Bejjani, A.; García-Granda, M.J.; Santos-Coquillat, A.; Ramos, V.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J. Toward Cell Selective Surfaces: Cell Adhesion and Proliferation on Breath Figures with Antifouling Surface Chemistry. ACS Appl. Mater. Interfaces 2016, 8, 6344–6353.
- Yabu, H.; Hirai, Y.; Kojima, M.; Shimomura, M. Simple fabrication of honeycomb-and pincushion-structured films containing thermoresponsive polymers and their surface wettability. Chem. Mater. 2009, 21, 1787–1789.
- De León, A.S.; de la Mata, M.; Molina, S.I. Hybrid hierarchically structured materials combining breath figures and thermal decomposition of KAuCl4. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126766.
- Li, Y.; Ma, X.; Ma, J.; Zhang, Z.; Niu, Z.; Chen, F. Fabrication of Pore-Selective Metal-Nanoparticle-Functionalized Honeycomb Films via the Breath Figure Accompanied by In Situ Reduction. Polymers 2021, 13, 316.
- Modigunta, J.K.R.; Male, U.; Huh, D.S. Formylated polystyrene for the fabrication of pore selective aldehyde group functionalized honeycomb patterned porous polystyrene films. J. Polym. Sci. B Polym. Phys. 2018, 56, 1181–1192.
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006.
- Cao, T.T.; Yabu, H.; Huh, D.S. Flower-like ordered porous array by combination of breath figure and layer-by-layer technique. Polymer 2021, 233, 124206.
- Zhang, L.; Li, X. Facile preparation of honeycomb-structured TiO2 nanofilm via breath figures assembly and coffee ring effect. Mater. Lett. 2018, 227, 74–77.
- Li, X.; Zhang, L.; Wang, Y.; Yang, X.; Zhao, N.; Zhang, X.; Xu, J. A Bottom-Up Approach To Fabricate Patterned Surfaces with Asymmetrical TiO2 Microparticles Trapped in the Holes of Honeycomblike Polymer Film. J. Am. Chem. Soc. 2011, 133, 3736–3739.
- Liu, Y.-Q.; Pan, G.-B.; Zhang, M.; Li, F. Micro-patterned composite films with bowl-like SnO2 microparticles. Mater. Lett. 2013, 92, 284–286.
- Shin, B.K.; Male, U.; Huh, D.S. In-situ pore filling of TiO2 nanoparticles in honeycomb patterned porous films: A modified breath figure method. Polymer 2018, 135, 1–8.
- Uyen Thi, P.N.; Male, U.; Huh, D.S. Fabrication of photo-responsive moth eye-like patterned poly (vinyl alcohol) films selectively containing TiO2 nanoparticles in the microdome. Polymer 2018, 144, 103–110.
- Li, X.; Wang, Y.; Zhang, L.; Tan, S.; Yu, X.; Zhao, N.; Chen, G.; Xu, J. Fabrication of honeycomb-patterned polyalkylcyanoacrylate films from monomer solution by breath figures method. J. Colloid Interface Sci. 2010, 350, 253–259.
- Cao, T.T.; Modigunta, J.K.R.; Male, U.; Huh, D.S. Pore-Selective SnS Functionalization in Honeycomb-Patterned Films by a Breath Figure Process Accompanied by Chemical Reaction. Adv. Mater. Interfaces 2018, 5, 1801174.
- Male, U.; Shin, B.K.; Huh, D.S. Polyaniline decorated honeycomb-patterned pores: Use of a reactive vapor in breath figure method. Polymer 2017, 121, 149–154.
- Cao, T.T.; Male, U.; Huh, D.S. Fabrication of pore-selective carboxyl group functionalized polyimide honeycomb-patterned porous films using KOH humidity. Polymer 2018, 153, 86–94.
- Modigunta, J.K.R.; Kim, J.M.; Cao, T.T.; Yabu, H.; Huh, D.S. Pore-selective modification of the honeycomb-patterned porous polystyrene film with poly(N-isopropylacrylamide) and application for thermo-responsive smart material. Polymer 2020, 201, 122630.
- Falak, S.; Shin, B.K.; Huh, D.S. Single-Step Pore-Selective Silver-Functionalized Honeycomb-Patterned Porous Polystyrene Film Using a Modified Breath Figure Method. Macromol. Res. 2021, 29, 519–523.
- Cao, T.T.; Uyen Thi, P.N.; Male, U.; Huh, D.S. Selective Coating of SnS on the Bio-Inspired Moth-Eye Patterned PEDOT: PSS Polymer Films. Macromol. Mater. Eng. 2019, 304, 1800727.
- Kim, J.M.; Falak, S.; Huh, D.S. Thermo-responsive release of rhodamine B in the pore-selective poly(N-isopropylacrylamide) immobilized honeycomb-patterned porous film. Polym. Bull. 2021, 79, 1911–1928.
- De León, A.S.; Molina, M.; Wedepohl, S.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J.; Calderón, M. Immobilization of Stimuli-Responsive Nanogels onto Honeycomb Porous Surfaces and Controlled Release of Proteins. Langmuir 2016, 32, 1854–1862.
- De León, A.S.; del Campo, A.; Rodríguez-Hernández, J.; Muñoz-Bonilla, A. Switchable and pH responsive porous surfaces based on polypeptide-based block copolymers. Mater. Des. 2017, 131, 121–126.
- Jiang, T.; Moghaddam, S.Z.; Thormann, E. A pH-responsive polyelectrolyte multilayer film with tunable interfacial properties. Polymer 2021, 214, 123367.
- Falak, S.; Shin, B.K.; Yabu, H.; Huh, D.S. Fabrication and characterization of pore-selective silver-functionalized honeycomb-patterned porous film and its application for antibacterial activity. Polymer 2022, 244, 124646.
- Chen, S.; Gao, S.; Jing, J.; Lu, Q. Designing 3D Biological Surfaces via the Breath-Figure Method. Adv. Healthc. Mater. 2018, 7, 1701043.
- Cao, T.T.; Yabu, H.; Huh, D.S. Hierarchical Nano/Micro Moth Eyelike Polymer Film Using Solid/Liquid Interfacial Reaction at Room Temperature. Langmuir 2020, 36, 9064–9073.
- Huang, C.; Shen, X.; Liu, X.; Chen, Z.; Shu, B.; Wan, L.; Liu, H.; He, J. Hybrid breath figure method: A new insight in Petri dishes for cell culture. J. Colloid Interface Sci. 2019, 541, 114–122.
- Rarima, R.; Unnikrishnan, G. Porous poly (lactic acid)/nano-silver composite membranes for catalytic reduction of 4-nitrophenol. Mater. Chem. Phys. 2020, 241, 122389.




