Recent developments in the field of the breath figure (BF) method leading to pore-selective functionalization of honeycomb-patterned (HCP) films attracted great interest. The pore-selective functionalization of the HCP film gives unique properties to the film which can be used for specific applications like protein recognition, catalysis, selective cell culturing, and drug delivery.
- self-assembly
- modified breath figure method
- interfacial reaction
- honeycomb-patterned porous film
- pore-selective functionalization
1. Introduction
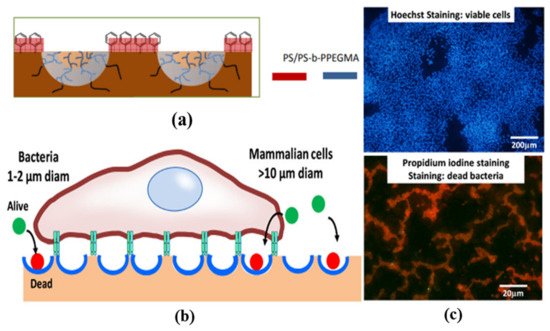
The functionality exclusive to the surface of the pores enables the use of these arrays in technical applications, such as the site-specific immobilization of proteins, essential to avoid hampering the activity of the protein which is beneficial to the specific selective adsorption of protein [43,88]. The protein orientation plays a critical role if protein microarrays are to be used as quantitative tools in biomedical research or clinically important protein-based nanomedicines [89]. For example, the surfaces prepared could be of potential interest as 3D cell culture platforms, that have been otherwise obtained via tedious multi-step procedures using lithographic techniques. The cell adhesion and proliferation on HCP films can be controlled by polymer film surface chemistry, which can be directed into the pores by functionalizing the pore-interior with glycopolymers or proteins [48,90,91]. This can enhance their proliferation and retain spherical morphology. Moreover, the specific patterning of metal or semiconductor materials could be applied in photonics, since the photonic band structure depends on the spatial periodic structures and the refractive indices of the dielectric materials in the structure [92].
2.1. Pore-Selective Functionalization of HCP Films by Self-Assembly
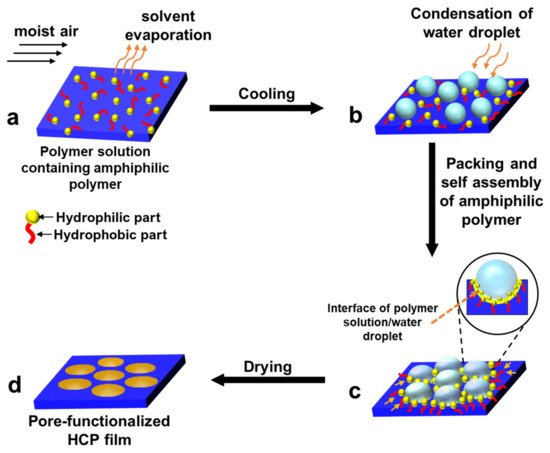
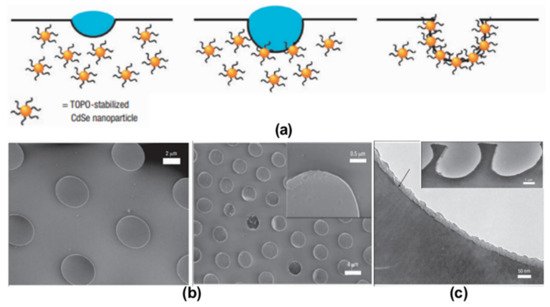
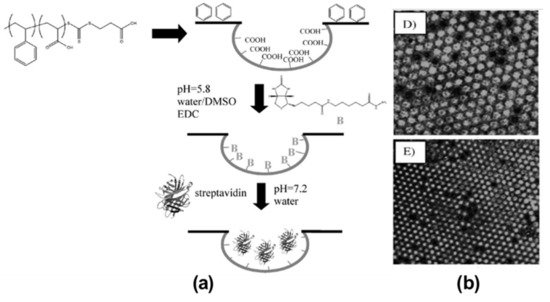
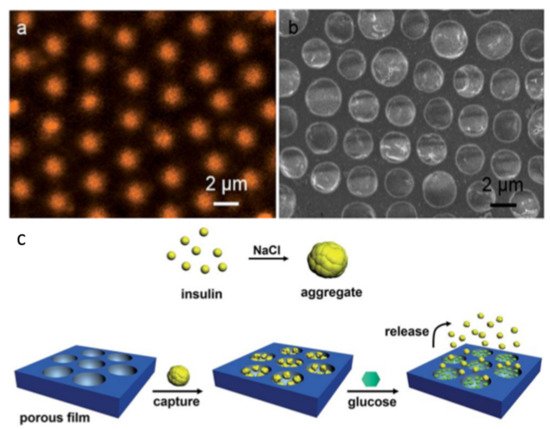
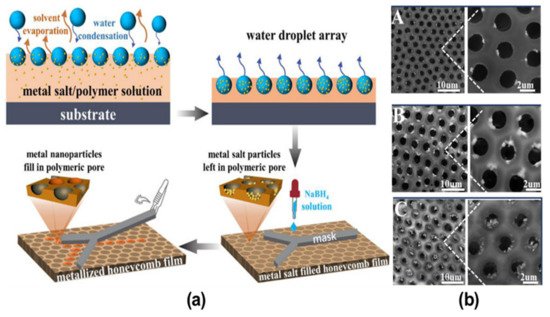
2.2. Pore-Functionalization by Self-Assembly Followed by Additional Treatment
2.3. Pore-Selective Functionalization of HCP Films Accompanying Chemical Reaction
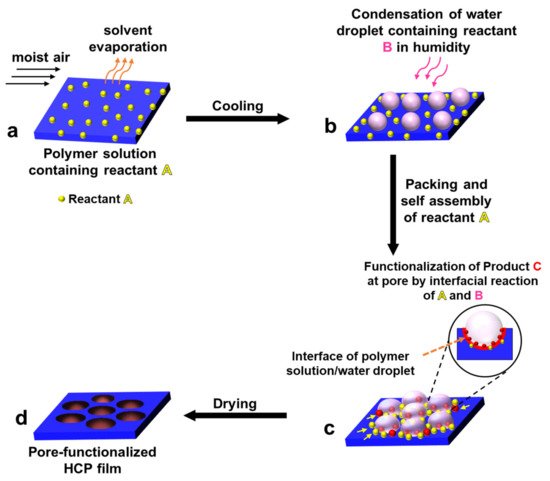
2.3.1. Pore-functionalization by BF process using water as a reactant
For the method, a component reactive to water was added in the polymer solution such as titanium chloride, [101,102] or tin chloride, [103] and cast under humid conditions. This favored a chemical reaction to happen at the aqueous droplet/polymer solution interface and resulted in their respective products at the pores as shown in Figure 12. Li et al. fabricated composite film with hemispherical or mushroom-like titanium oxide (TiO2) microparticles lying in the pores of an HCP PS film which can be used with or without the polymer matrix as shown in Figure 13. Similarly, Shin et al. reported the fabrication of PS HCP films with TiO2 NPs filled pores with slight modification in the BF process. [104] Here, the micro-water droplets were used as a reactive template, and titanium butoxide (Ti(C4H9O)4) was poured into the partially dried PS solution during the BF process. The (Ti(C4H9O)4) reacts with micro-water droplets and forms TiO2 NPs in the pores shown in Figure 14. In most of these reports, the aqueous droplets were used as micro-reactors for the preparation of metal hydroxides/oxides with specific shapes but not for the functionalization of pores in HCP film, given in Table 2. Like, using it as template for the fabrication of microdome selectively containing TiO2 NPs which may have potential applications in different fields of electronic optoelectronic, and anti-reflective surfaces. [105] In another example, water droplets were used as polymerization initiators for the polymerization of acrylate monomer to obtain polyacrylate HCP film. [106] The aggregation of polymer around the water droplets triggered the polymerization of alkylcyanoacrylate, and the –OH heads of the formed chains were anchored on the water droplets and the growing chains persisted at the water–solution interface forming a pore-selective HCP film as shown in Figure 15.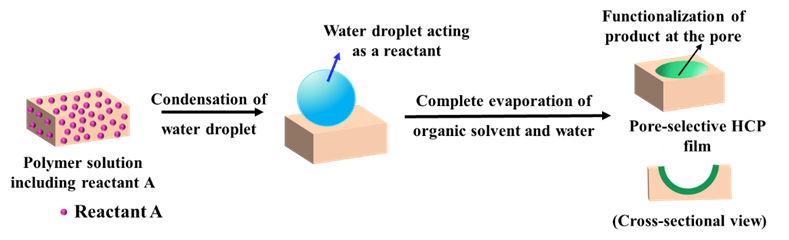
Figure 12. Pore-selective functionalization of HCP films by using water as a reactant. The polymer solution containing a reactant reactive to water is cast under humid conditions for the reaction to occur at the interface of polymer solution/water droplet to achieve pore-selective functionalization.
Table 2. Summary of reports using one reactant in polymer solution and water from the humid atmosphere as another reactant.
|
Reactant A in the Polymer solution |
Humid conditions |
Product/Functionalization in pore |
Ref. |
|
Titanium tetrachloride |
Water |
Titanium dioxide |
[101] |
|
Titanium tetrachloride |
Water |
Titanium dioxide |
[102] |
|
Tin tetrachloride |
Water |
Tin dioxide |
[103] |
|
Titanium butoxide |
Water |
Titanium dioxide NPs |
[104] |
|
Titanium n-butoxide |
Water |
Titanium dioxide |
[107] |
|
Titanium tetraisopropoxide |
Water |
Titanium dioxide |
[108] |
|
Alkylcyanoacrylate |
Water |
PACA |
[106] |
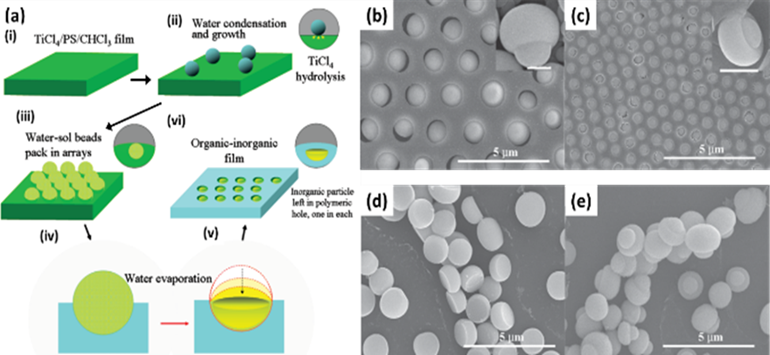
Figure 13. (a) Scheme for the formation of highly ordered asymmetrical inorganic particle/polymer composite films by BFs method. (b, c) SEM images of the obtained composite film from the TiCl4/ PS/CHCl3 solution with different concentrations of TiCl4, SEM images of (c) hemispherical and (d-e) mushroom-like TiO2 microparticles after calcination of the composite film at 450 °C for 3h. Reprinted with permission from ref [102]. Copyright 2011 Journal of the American Chemical Society.
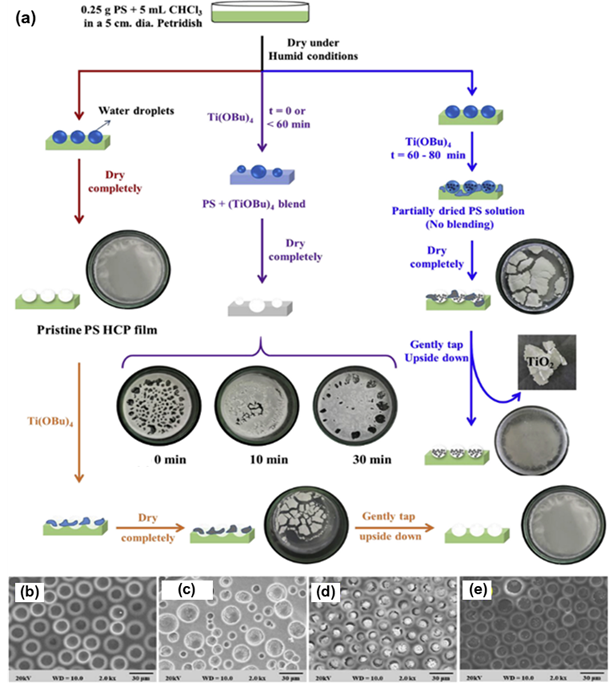
Figure 14. (a) Schematic representation showing different reaction conditions with the variance in time intervals of pouring titanium butoxide solution to the PS solution. (b-e) shows the typical SEM images of PS film obtained at a different time interval. Reprinted with permission from ref [104]. Copyright 2018 Polymer.
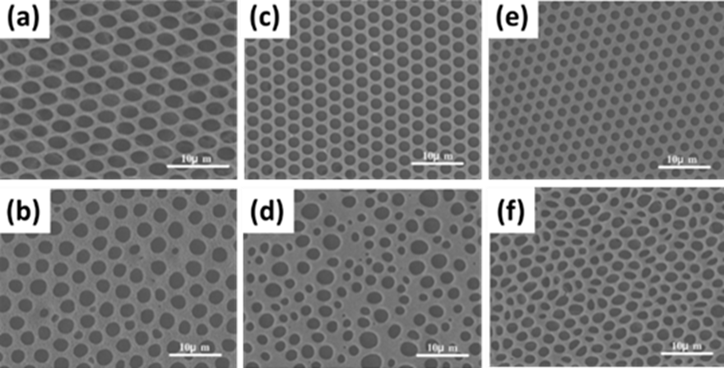
Figure 15. SEM images of porous PACA films fabricated from monomer solutions of (a) ECA, (c) BCA, (e) OCA, and polymer solutions of (b) PECA, (d) PBCA, (e) POCA. Reprinted with permission from ref [106]. Copyright 2010 Journal of Colloid and Interface Science.
2.3.2. BF process accompanying chemical reactions with a non-aqueous reactant
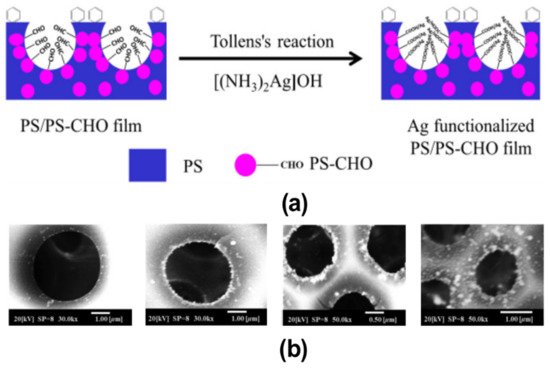
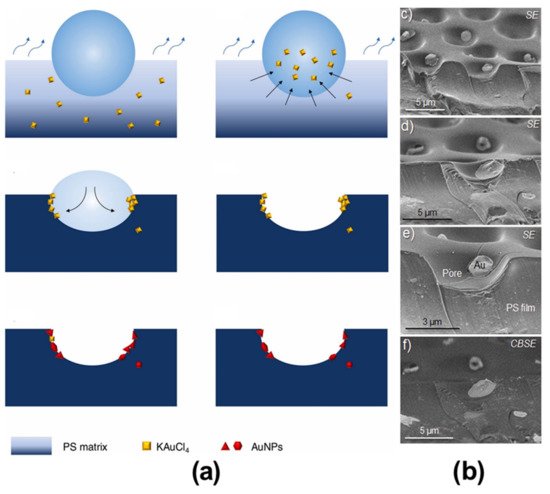
In the bottom-up process of the BF mechanism, the stage at which there is an interfacial interaction of water droplet and polymer solution can be applied for the site-selective functionalization. Applying this phenomenon, Huh et al. recently introduced a modified BF method, called the reactive BF (rBF) method, for the single-step fabrication of pore-selective functionalized films by accompanying an interfacial chemical reaction during the BF process. [98,109-113] For this method, a solvent-soluble reactant is added to the hydrophobic polymer solution, and a water-soluble counter reactant is added to the water used for humid conditions. When the polymer solution containing functionalizing reactant is cast under humid conditions containing counter reactant, a chemical reaction happens at the aqueous droplet/polymer solution interface and the product is formed in the pore of HCP film as a coating. [109-112] The benefit of this approach is that any material, either organic [109-112] or inorganic [113] can be functionalized by this method in one single step. Male et al. fabricated pore functionalized polyaniline (PANI) HCP porous films by in-situ polymerization via the BF method. [110] They added benzoyl peroxide to the PS solution and cast under reactive vapor of aniline hydrochloride, which resulted in PANI functionalized pores which otherwise might be impossible due to poor solubility of PANI in most of the solvents, as shown in Figure 16. Cao et al. fabricated a pore-selective carboxyl group functionalized polyimide HCP porous films using potassium hydroxide as a counter reactant in humidity as shown in Figure 17. [111] Followed by treating with Tollen’s reagent for the decoration of Ag NPs inside the pores. Similarly, tin sulfide was functionalized in the pores of PS HCP porous films using H2S in the humidity as the reacting agent, shown in Figure 18. [109] The pore-selective coating of SnS was used as a template for the fabrication of moth-eye patterned film for antireflecting due to photo-responsive property under solar stimulated light illumination. [114] Modigunta et al. fabricated a temperature-sensitive polymer, poly (N-isopropyl acrylamide) (PNIPAAm) inside the pores of the HCP porous films using this same strategy. They oxidized the PS-CHO assembled inside the pores of HCP porous films using oxone to fabricate carboxylic acid (-COOH) in the pores of the HCP porous films. [112] The oxidization of the aldehyde group occurred at the interface of the water droplet/polymer solution accompanying an interfacial reaction. This PS-pf-COOH film was functionalized with amine-terminated PNIPAAm (NH2-PNIPAAm) via EDC coupling as shown in Figure 19. Later, they showed capturing of Ag particles in the pores of the HCP film at the LCST of PNIPAAm. This opens a plethora of prospects to immobilize various types of functional or smart materials containing hydroxyl or amine groups. The fabrication of pore-selective –COOH functionalized HCP films by the rBF method may have various applications, like controlled drug release [115,116] or biosensors [117] because the –COOH group functionalized at the pores can be further immobilized with various types of functional or smart materials containing hydroxyl or amine groups. [112,118] Recently, Falak et al. fabricated HCP porous PS films with pore-selective Ag using the rBF method accompanied by an interfacial reaction shown in Figure 20. [119] In this study, an inorganic reactant AgNO3 was added in the aq. humidity as a functionalizing agent. On the other hand, ferrocene was included in the PS polymer solution as a reducing agent, which reduced AgNO3 to Ag inside the pores of the HCP films. This strategy might be useful for 3D micro-patterning of biological moieties of interest to the pores for applications in tissue engineering, antibacterial membranes, protein- and cell-based biosensors, microelectronic devices, or filtration membranes. [119]
Table 3. Summary of reports using two reactants, one in the polymer solution and the other in a humid atmosphere for the functionalization of pores in HCP films.
|
Reactant A in the Polymer solution |
Reactant B in Humid conditions |
Functionalization in pore (Product C) |
Ref. |
|
Poly (4-vinylpyridine) |
Formic acid |
PVP-FA precipitate |
[120] |
|
Benzoyl peroxide |
Aniline |
Polyaniline |
[110] |
|
Tin dichloride |
Hydrogen sulfide |
Tin sulfide |
[109] |
|
Polyimide |
Potassium hydroxide |
Carboxylic group |
[111] |
|
PS aldehyde (PS-CHO) |
Oxone |
Carboxylic acid (-COOH) |
[112] |
|
Ferrocene |
Silver Nitrate |
Silver |
[119] |
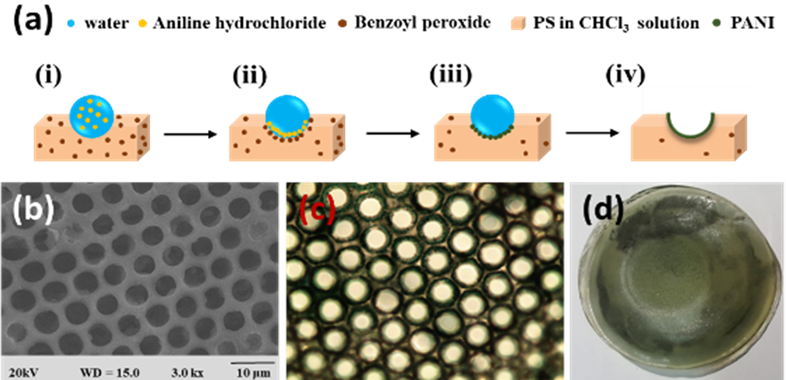
Figure 16. (a) Mechanism showing the formation of PANI-f-PS film with PANI functionalized pores: (i) Condensed aq. aniline hydrochloride droplets on PS-BPO solution during BF process, (ii) assembly of aniline hydrochloride and BPO at aqueous droplet/polymer solution interface, (iii) interfacial polymerization, (iv) and formation of PANI functionalized pores after completion of reactive BF process. (b) SEM, (c) optical, and (d) digital photograph of PANI-f-PS film. Reprinted with permission from ref [110]. Copyright 2017 Polymer.
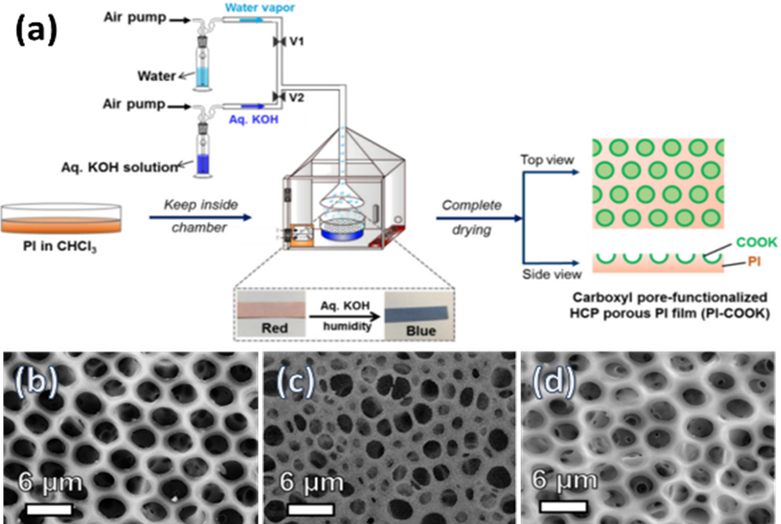
Figure 17. (a) Schematic representation showing the fabrication of the pore-selective carboxyl functionalized PI HCP films (PI-COOK) via BF method under aq. KOH humidity with a time interval after using initial water humidity. SEM images of PI films fabricated under (b) water humidity, (c) aq. KOH humidity, and (d) under aq. KOH humidity for 20 min after initial water humidity. Reprinted with permission from ref. [111] Copyright 2018 Polymer.
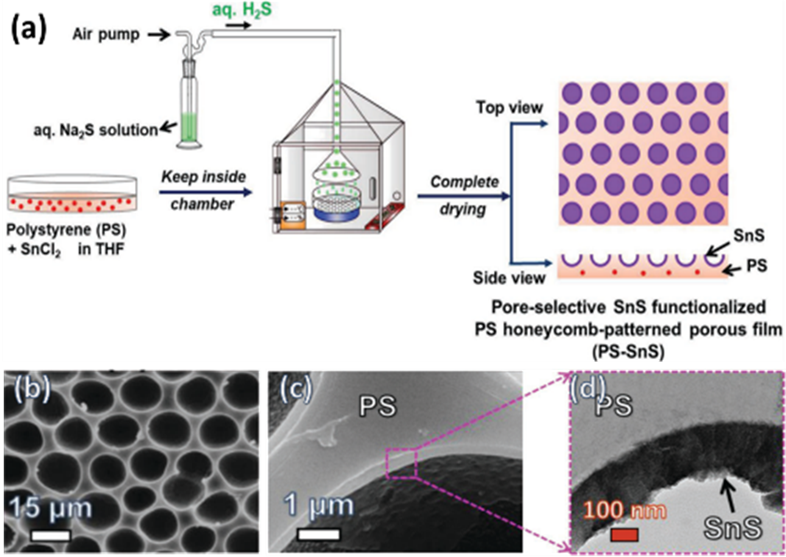
Figure 18. (a) Schematic representation showing the experimental process for the pore-selective SnS-functionalized PS HCP film using a modified breath figure method. SEM images of PS HCP films fabricated by casting PS solution containing SnCl2 under (b, c) aq. H2S humidity, and (d) magnified TEM image showing a thin layer of SnS. Reprinted with permission from ref [109]. Copyright 2018 Advanced Materials Interfaces. (e) SEM image of PEDOT: PSS-SnS MEP film with SnS thin layer coated on the microdomes for the application of an antireflecting film. Reprinted with permission from ref. [112] Copyright 2019 Macromolecular Materials and Engineering.
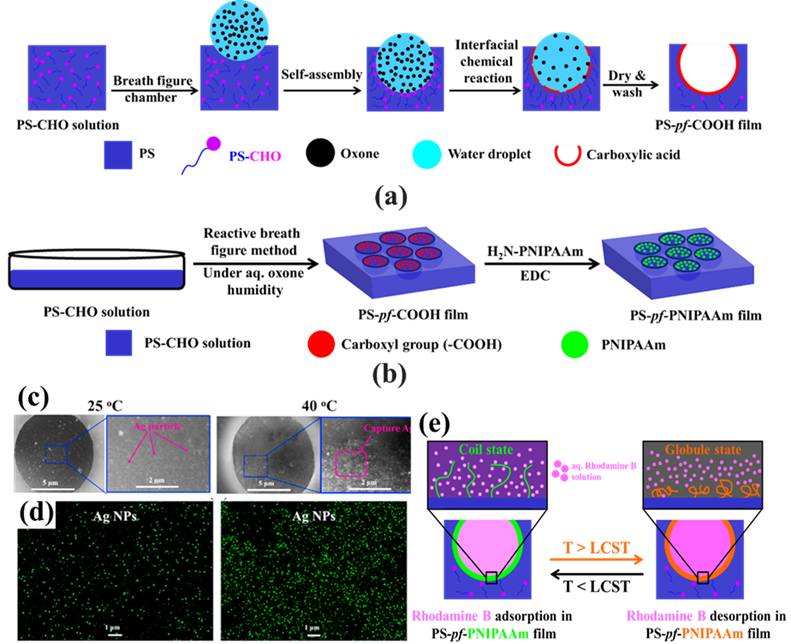
Figure 19. (a) The possible mechanism involved in the rBF process via self-assembly process accompanying an interfacial chemical reaction between formylated PS and oxone at the interface of the water droplet/polymer solution. (b) Schematic process for the preparation of pore-selective PNIPAAm modified (PS-pf-PNIPAAm) film by the reaction between PS-pf-COOH film and an aq. H2N-PNIPAAm solution in the presence of EDC. (c-d) SEM images and elemental mapping show the capture of Ag particles at 25 °C and 40 °C. Reprinted with permission from ref. [112] Copyright 2020 Polymer. (e) Scheme showing the release of rhodamine B at the LCST of PNIPAAm. Reprinted with permission from ref. [115] Copyright 2021 Polymer Bulletin.
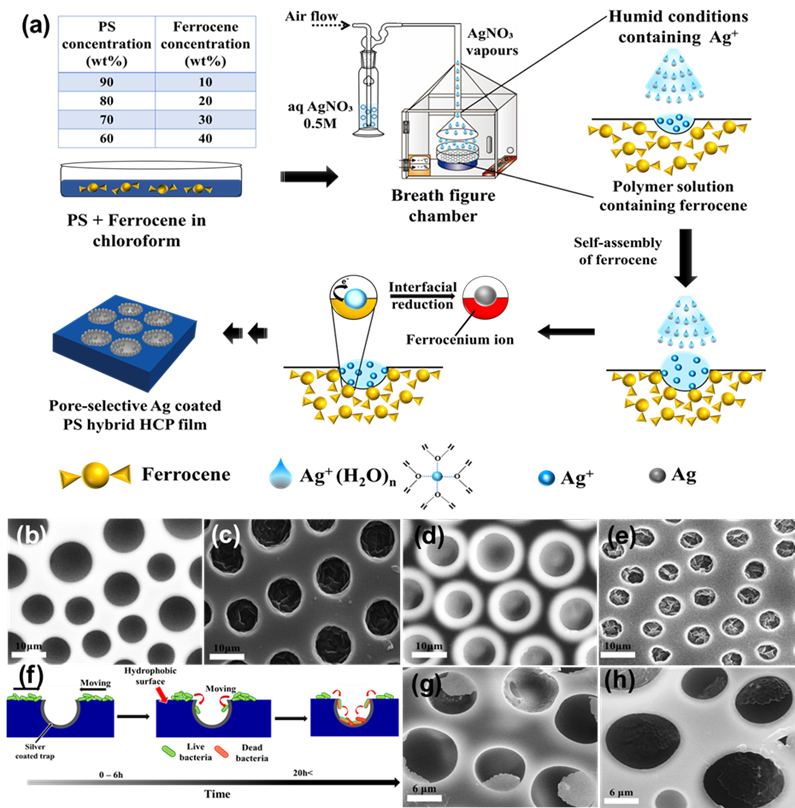
Figure 20. (a) Schematic representation showing the fabrication of the pore-selective Ag functionalized PS HCP films via rBF method under aq. AgNO3 humidity. Typical SEM images of the 10 and 20 wt% PS HCP films fabricated under (b,d) aq. humidity, and (c,e) aq. AgNO3 humidity, respectively. (f) The mechanism for trapping of bacteria inside the pits of the Ag-functionalized porous HCP film. SEM images of (g) E. coli and (h) S. aureus showing the trapped bacteria at the pores of the HCP film. Reprinted with permission from ref. [119] Copyright 2022 Polymer.
Since the discovery of the BF method in 1994, the past decade has seen rapid developments in the BF approach. HCP films fabricated via the BF method have shown great potential in chemistry and materials science. As the porous films are composed of the polymer framework and pores, their use can be applicable to frameworks and pores. [121] Compared with the vigorous progress of the framework, the applications of pores is less reported, acting as secondary templates, [105,114,122,123] selective antibacterial surfaces, [5,113,124] catalysis, [70,71] selective cell culturing, [5,124] drug capture and release [95,115] or in protein recognition [87,89] as shown in Figure 21. Thus, how to develop the new functions of the cavities becomes very meaningful, which will bring new vitality to the field of porous films.
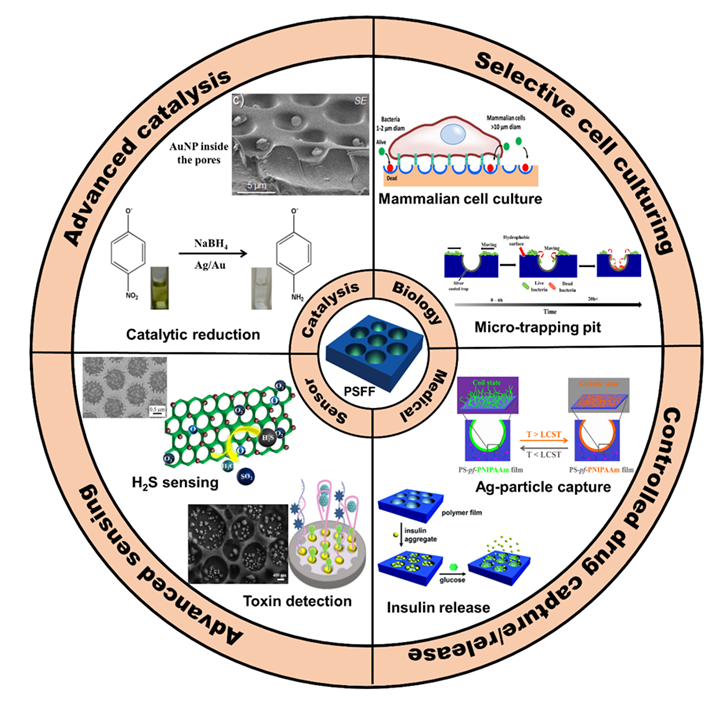
Figure 21. Pore-selectively functionalized HCP films via the modified BF method and their applications in biology, medical, sensor application, and catalysis. PSFF- Pore-Selectively Functionalized Film.
3. Conclusion
The functionalization of pore surfaces in one-step is possible by using the dynamic BF processes, in which there is an active flow of vapors over the surface of the polymeric solution facilitating the evaporation of water droplets containing the chemical moieties dissolved in it. Thereby, facilitating the interfacial reaction between the two reactants at the interface of polymer/ water droplet and functionalization of pore surfaces. However, in the static BF process, the effects of vapor flow could be neglected due to the passive exposure of polymeric solutions without inducing extra vapor flow. Therefore, pore-selective functionalization using the self-assembly of amphiphilic polymers is feasible with the static BF method while pore-selective functionalization using the self-assembly accompanying the interfacial chemical reaction between two reactants is not achievable.
Earlier, optimizing versatility in functionalizing these pore surface structures required an ex situ process, and the in situ functionalization is limited to surfactants that are compatible with the BF formation. Therefore, the possibility of combining the BF method with interfacial chemical reactions might push forward the development of pore-selective functionalization in both fundamental and application aspects. Although the principle of the BF method looks very simple, optimizing the concentration of reactants for the formation of products at the pores without compromising the pattern remains a challenge. In the modified BF method, the interfacial interactions between water droplets containing a reactant and the polymer solution containing the counter reactant play a key role. Understanding the kinetics and conditions of the chemical reaction is one of the most important aspects. Additionally, the selection of reactants concerning their nature, solubility, or other aspects will help fabricate HCP films with the desired functionalization in a more controllable way. The traces of reactant or the irrelevant byproducts left at the pores may also be unfavorable in this system. Moreover, the chemical reactions possible for the pore-selective functionalization are limited to the formation and application of the new products formed. The fabrication of pore selective COOH opens up a plethora of opportunities for further immobilization of the smart or functional polymers, proteins, ligands, and biomolecules which extends the applications of the HCP films fabricated via the modified BF method.
This entry is adapted from the peer-reviewed paper 10.3390/nano12071055
