
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hidekatsu Yanai | -- | 2640 | 2022-04-04 04:05:37 | | | |
| 2 | Bruce Ren | + 1 word(s) | 2641 | 2022-04-06 03:01:47 | | |
Video Upload Options
Several randomized, double blind, placebo-controlled trials (RCTs) have demonstrated that low-density lipoprotein cholesterol (LDL-C) lowering by using statins, including high-doses of strong statins, reduced the development of cardiovascular disease (CVD). However, among the eight RCTs which investigated the effect of statins vs. placebos on the development of CVD, 56–79% of patients had the residual CVD risk after the trials. In three RCTs which investigated the effect of a high dose vs. a usual dose of statins on the development of CVD, 78–87% of patients in the high-dose statin arms still had the CVD residual risk after the trials. An analysis of the characteristics of patients in the RCTs suggests that elevated triglyceride (TG) and reduced high-density lipoprotein cholesterol (HDL-C), the existence of obesity/insulin resistance, and diabetes may be important metabolic factors which determine the statin residual CVD risk.
1. Introduction
2. Properties and Normal Metabolism of Lipoproteins
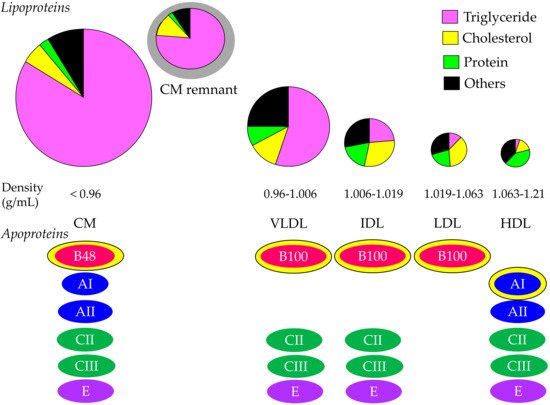
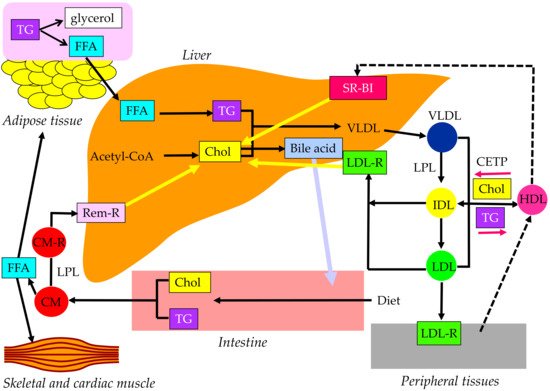
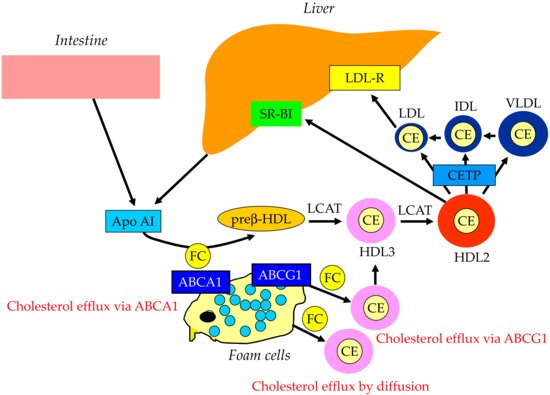
3. What Is the Statin Residual CVD Risk?
| Primary Prevention | Secondary Prevention | |||||||
|---|---|---|---|---|---|---|---|---|
| Trials | WOSCOPS [2] | ASCOT-LLA [3] | AFCAPS/ TexCAPS [4] |
CARDS [5] | JUPITER [6] | 4S [1] | LIPID [7] | CARE [8] |
| Used statins and daily dose | pravastatin 40 mg | atorvastatin 10 mg | lovastatin 20–40 mg | atorvastatin 10 mg | rosuvastatin 20 mg | simvastatin 20 mg | pravastatin 40 mg | pravastatin 40 mg |
| Follow-up (years) | 4.9 | 3.3 | 5.2 | 4 | 1.9 | 5.4 | 6.1 | 5 |
| Risk reduction of cardiovascular events (statins vs. placebo, %) | 31 | 21 | 37 | 37 | 44 | 35 | 29 | 24 |
| Risk reduction of cardiovascular events/year (%) | 6.3 | 6.4 | 7.1 | 9.3 | 23.2 | 6.5 | 4.8 | 4.8 |
| Residual CVD risk (%) | 69 | 79 | 63 | 63 | 56 | 65 | 71 | 76 |
| Residual CVD risk/year (%) | 93.7 | 93.6 | 92.9 | 90.8 | 76.8 | 93.5 | 95.2 | 95.2 |
| Achieved serum lipid levels | ||||||||
| LDL-C (mg/dL) | 142 | 90 | 115 | 81 | 55 | 122 | 113 | 97 |
| HDL-C (mg/dL) | 46 | 51 | 39 | 49 | 50 | 49 | 38 | NA |
| TG (mg/dL) | 142 | 114 | 143 | 143 | 99 | 119 | 126 | NA |
| Baseline data | ||||||||
| BMI (kg/m2) | 26 | 28.6 | 27.1 (men) 26.4 (women) |
28.7 | 28.4 | 26 | BMI > 30kg/m2, 18% | 28 |
| Prevalence of diabetes (%) | 1 | 24.3 | 6.8 | 100 | 0 | 5 | 9 | 14 |
| Trials | PROVE-IT TIMI22 [9] | IDEAL [10] | TNT [11] |
|---|---|---|---|
| Used statins and daily dose | pravastatin 40 mg vs. atorvastatin 80 mg | simvastatin 20 mg vs. atorvastatin 80 mg | atorvastatin 10 mg vs. atorvastatin 80 mg |
| Follow-up (years) | 2 | 4.8 | 4.9 |
| Risk reduction of cardiovascular events (high-dose vs. usual-dose, %) | 16 | 13 | 22 |
| Risk reduction of cardiovascular events/year (%) | 8 | 2.7 | 4.5 |
| Residual CVD risk (%) | 84 | 87 | 78 |
| Residual CVD risk/year (%) | 92 | 97.3 | 95.5 |
| Achieved serum lipid levels | |||
| LDL-C | 95 vs. 62 | 99.8 vs. 80 | 101 vs. 77 |
| HDL-C | 42 vs. 40 | 50.6 vs. 50.1 | apprpximately 48 vs. 49 |
| TG | 175.8 vs. 136.2 | 137.2 vs. 118.5 | apprpximately 140 vs. 160 |
| Baseline data | |||
| BMI (kg/m2) | 29.6 vs. 29.6 | 27.3 vs. 27.3 | 28.6 vs. 28.4 |
| Prevalence of diabetes (%) | 18 vs. 18 | 12 vs. 12 | 15 vs. 15 |
4. Current Views on Estimation of Residual Cardiovascular Risk in Patients Treated with Statins
References
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Shepherd, J.; Cobbe, S.M.; Ford, I.; Isles, C.G.; Lorimer, A.R.; MacFarlane, P.W.; McKillop, J.H.; Packard, C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 1995, 333, 1301–1307.
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158.
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622.
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696.
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; JUPITER Study Group; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207.
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357.
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009.
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504.
- Lindahl, C.; Szarek, M.; Tsai, J.; Incremental Decrease in End Points through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomized controlled trial. JAMA 2005, 294, 2437–2445.
- LaRosa, J.C.; Grundy, S.M.; Waters, D.D.; Shear, C.; Barter, P.; Fruchart, J.C.; Gotto, A.M.; Greten, H.; Kastelein, J.J.; Treating to New Targets (TNT) Investigators; et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N. Engl. J. Med. 2005, 352, 1425–1435.
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Cholesterol Treatment Trialists’ (CTT) Collaboration; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681.
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278.
- Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421.
- Buse, J.B.; Ginsberg, H.N.; Bakris, G.L.; Clark, N.G.; Costa, F.; Eckel, R.; Fonseca, V.; Gerstein, H.C.; Grundy, S.; Nesto, R.W.; et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007, 30, 162–172.
- Smith, S.C., Jr.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006, 113, 2363–2372.
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C., Jr.; Stone, N.J. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239.
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; De Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14 (Suppl. 2), E1–E40.
- Chait, A.; Ginsberg, H.N.; Vaisar, T.; Heinecke, J.W.; Goldberg, I.J.; Bornfeldt, K.E. Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes 2020, 69, 508–516.
- Briel, M.; Ferreira-Gonzalez, I.; You, J.J.; Karanicolas, P.J.; Akl, E.A.; Wu, P.; Blechacz, B.; Bassler, D.; Wei, X.; Sharman, A.; et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: Systematic review and meta-regression analysis. BMJ 2009, 338, b92.
- Ueshima, K.; Itoh, H.; Kanazawa, N.; Komuro, I.; Nagai, R.; Takeuchi, M.; Yamazaki, T.; EMPATHY Study Group. Rationale and Design of the Standard versus Intensive Statin Therapy for Hypercholesterolemic Patients with Diabetic Retinopathy (EMPATHY) Study: A Randomized Controlled Trial. J. Atheroscler. Thromb. 2016, 23, 976–990.
- Tada, H.; Kawashiri, M.; Nomura, A.; Yoshimura, K.; Itoh, H.; Komuro, I.; Yamagishi, M. Serum triglycerides predict first cardiovascular events in diabetic patients with hypercholesterolemia and retinopathy. Eur. J. Prev. Cardiol. 2018, 25, 1852–1860.
- Schwartz, G.G.; Abt, M.; Bao, W.; DeMicco, D.; Kallend, D.; Miller, M.; Mundl, H.; Olsson, A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015, 65, 2267–2275.
- Yanai, H.; Hirowatari, Y.; Yoshida, H. Diabetic dyslipidemia: Evaluation and mechanism. Glob. Health Med. 2019, 1, 30–35.
- Yanai, H.; Hirowatari, Y.; Ito, K.; Kurosawa, H.; Tada, N.; Yoshida, H. Understanding of diabetic dyslipidemia by using the anion-exchange high performance liquid chromatography data. J. Clin. Med. Res. 2016, 8, 424–426.
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450.




