
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Jesus | -- | 19480 | 2022-04-02 12:31:34 | | | |
| 2 | Honorina Cidade | -15677 word(s) | 3803 | 2022-04-29 20:22:29 | | | | |
| 3 | Ana Jesus | + 9 word(s) | 3812 | 2022-04-29 23:07:35 | | | | |
| 4 | Vivi Li | -24 word(s) | 3788 | 2022-05-05 05:46:37 | | | | |
| 5 | Vivi Li | Meta information modification | 3788 | 2022-05-05 05:47:00 | | |
Video Upload Options
The use of sunscreens is a recommended practice to protect skin from solar-induced damage. Around 30 UV filters can be used in sunscreen products in the European Union. However, low photostability and putative toxicity for humans and environment have been reported for some UV filters. Therefore, it is important to develop new UV filters with improved safety profile and photostability. Over the last two decades, nearly 200 new compounds have revealed promising photoprotection properties. The explored compounds were obtained through different approaches, including exploration of natural sources, synthetic pathways, and nanotechnology. Almost 50 natural products and around 140 synthetic derivatives have been studied aiming the discovery of novel, effective, and safer future photoprotective agents.
1. Introduction
2. Challenges
3. Prospects
3.1. Nature as a Source of Potential Photoprotective Agents and UV Filters
| Organism and Species | Main Identified Secondary Metabolites | Activity | Values | References |
|---|---|---|---|---|
| Botanical Extracts and Metabolites | ||||
| Methanolic extract of grape seeds (from Village Farm and Winery; Nakhon Ratchasima, Thailand) | (+)-catechin (36) and (-)-epicatechin (37) (determined by HPLC)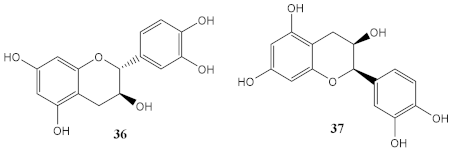 |
Photoprotective (% cell viability) | At 25 μg/mL 10 J/cm2 (110%) 20 J/cm2 (68%) |
[33] |
| Photodegradation | 36 = 35.1%; 37 = 31.3% Combination with UV filter: 36 (4.6%); 37 (7.0%) |
|||
| Hydroethanolic extract of Vitis vinifera L. | Flavonoids, phenolic compounds, procyanidins, among others (determined by HPLC) | Antioxidant (DPPH) at 1mg/mL | 707.00 ± 0.03 µmol/g (pH = 5) 1098.00 ± 0.01 µmol/g (pH = 7) |
[34] |
| Photoprotection | SPF = 20–76 λc = 360–381 nm (pH = 5) |
|||
| Ethanolic commercial extract of olive leaves | 20% of oleuropein (38) |
Antioxidant (DPPH) | 38: IC50 = 11.75 ± 1.01 μg/mL Extract: IC50 = 13.8 ± 0.8 μg/mL |
[35] |
| Photoprotective | λmax = 376 nm SPF = 22 |
|||
| Ethanolic Extract of varied Lippia species (L. brasiliensis, L. rotundifolia, L. rubella and L. sericea) | Phenols and flavonoids | Antioxidant (DPPH) | IC50 = 0.604 mg/mL | [36] |
| Photoprotective | SPF = 1.7–7.6 (formulation with 10% of the extract) λc = 375 nm |
|||
| Ethanolic extract of Amazonian Cecropia obtusa leaves | Polyphenols | Antioxidant | IC50 = 1.63 µg/mL (DPPH) IC50 = 0.34 µg/mL(O2−) IC50 = 0.55 µg/mL(1O2) |
[37] |
| Photoprotective | SPF = 16 | |||
| Cytotoxicity (HaCaT keratinocyte cell line) | At 20 µg/mL: cell viability = 100% | |||
| Ethanolic extract of Acacia catechu heartwood | - | Photoprotective | SPF = 24–30 | [38] |
| Hydroalcoholic extract of five wild Brazilian bamboo species (Chusqueaspp., Aulonemia aristulata, and Merostachys pluriflora) | Phenolic compounds | Antioxidant (DPPH) | IC50 = 137.55–260 μg/mL | [39] |
| Photoprotective | SPF (before irradiation) = 34–86 SPF (after irradiation) = 14–44 |
|||
| Dichloromethane/acetone (1:1) extract from Lasallia pustulata | Lichenic metabolites, being gyrophoric acid (39) identified by HPLC  |
Antioxidant (DPPH) | 25 % at 500 µg/mL | [40] |
| Photoprotective | λmax = 300 nm SPF = 5.03 |
|||
| Cytotoxicity (HaCaT keratinocytes cell line) | IC50 = 168 ± 33 µg/mL (before radiation) IC50 > 200 µg/mL (after radiation) |
|||
| Wood powder | - | Photoprotective | SPF = 11 (formulation) SPF = 37 (formulation + 5% of wood powder) |
[41] |
| Ethanolic extracts of Alpinia galanga, Curcuma longa and Aloe vera | Flavonoids, phenols and terpenoids | Photoprotective | SPF = 18.2 (extract of C. longa) λmax = 290 nm (C. longa) SPF = 15.1 (A. galanga) λmax = 290 nm (A. galanga) |
[42] |
| Coconut oil | High quantity of saturated fatty acids | Photoprotective | λmax = 205 nm (coconut oil) λmax = 320 nm (coconut oil + BP-3) |
[43] |
| Resveratrol (40) and ethanolic extract of green tea | Resveratrol (40)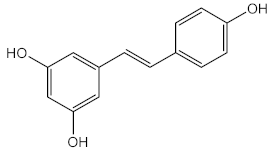 |
Antioxidant (DPPH) | IC50 = 38.67–85.44 % (resveratrol) IC50 = 37.41–77.50 % (green tea extract) |
[44] |
| Photoprotective | λmax = 310 nm (40) λmax = 270 nm (green tea) SPF = 9.35 (40) SPF = 14.59 (green tea extract) SPF = 16.91 (40 and green tea extract) |
|||
| Marine Organisms Extracts and Metabolites | ||||
| Methanolic extract of red macroalgae Curdiea racovitzae and Iridaea cordata | MAAs, with major quantity of palythine (41), asterina-330 (42), and shinorine (43)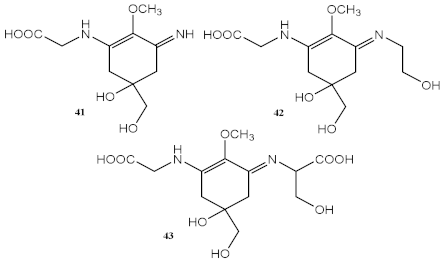 |
Antioxidant (DPPH) | IC50 = 970.00 μg/mL (C. racovitzae) IC50 = 2960.00 μg/mL (I. cordata) |
[45] |
| Photoprotective | λmax = 320 nm (both) λc = 356 nm (C. racovitzae) λc = 347 nm (I. cordata) |
|||
| Cytotoxicity (HaCaT keratinocytes cell line) | At 1 mg/mL % cell viability = 89 (C. racovitzae) % cell viability = 73 (I. cordata) |
|||
| Ethanolic extract of brown macroalgae Sargassum cristafolium | Palythine (41) | Photoprotective | λc = 370 nm | [49] |
| Methanolic extract red alga Corallina pilulifera | - | Antioxidant (DPPH) | At 200 mg/mL: 80% scaveging activity | [50] |
| Metabolite from extracts of cyanobacteria Stigonema sp., Scytonema sp. and Lyngbya sp. | Scytonemin (44)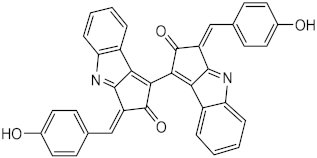 |
Photoprotective | λmax = 252, 278, 300, 386 nm | [51] |
| Metabolites from aqueous methanolic extract of cyanobacteria Microcystis aeruginosa | MAAs shinorine (43) and porphyra-334 (45)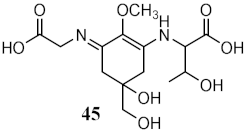 |
Photoprotective | λmax = 334 nm | [52] |
| Metabolites from ethyl acetate extract of marine fungi Penicillium echinulatum | Quinolinic Alkaloids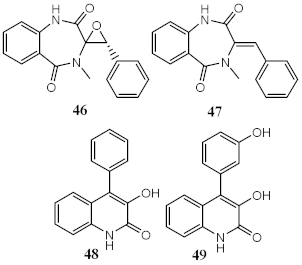 |
Photoprotective | λmax = 287 (48) λc = 335 nm (48) λmax = 330 (49) λc = 334 nm (49) |
[53] |
| Phototoxicity (HaCaT keratinocytes cells) | Reduction of ROS (43%) at 200 µg/mL (49) | |||
| Metabolites from dichloromethane/methanol (2:1) extract of algae Bostrychia radicans -associated fungi Annulohypoxylon stygium | 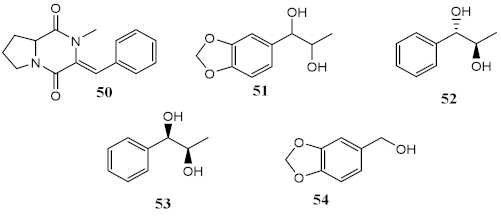 |
Phototoxicity (3T3 murine fibroblasts) | PIF = 1.00 (50 and 51) PIF = 5.2 (54) |
[54] |
| Metabolite from ethanolic extract of plant Thalassia testudinum | Thalassiolin B (55)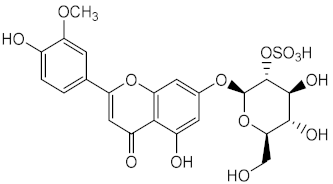 |
Antioxidant (DPPH) | IC50 = 100 μg/mL | [55] |
| Repair of Acute UVB-Damaged Skin | Skin damage suppression (with 55 at 240 μg/cm2) = 90% | |||
| Platyfish Xiphophorus metabolite | Melanin (56)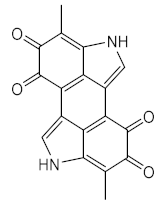 |
Photo-repair of the skin | Stimulate the production of melanin, which reduced the formation of pyrimidine dimers. | [56] |
3.2. Synthetic Derivatives with Photoprotective and UV Filter Activity
3.2.1. Inorganic UV Filters
3.2.2. Organic UV Filters
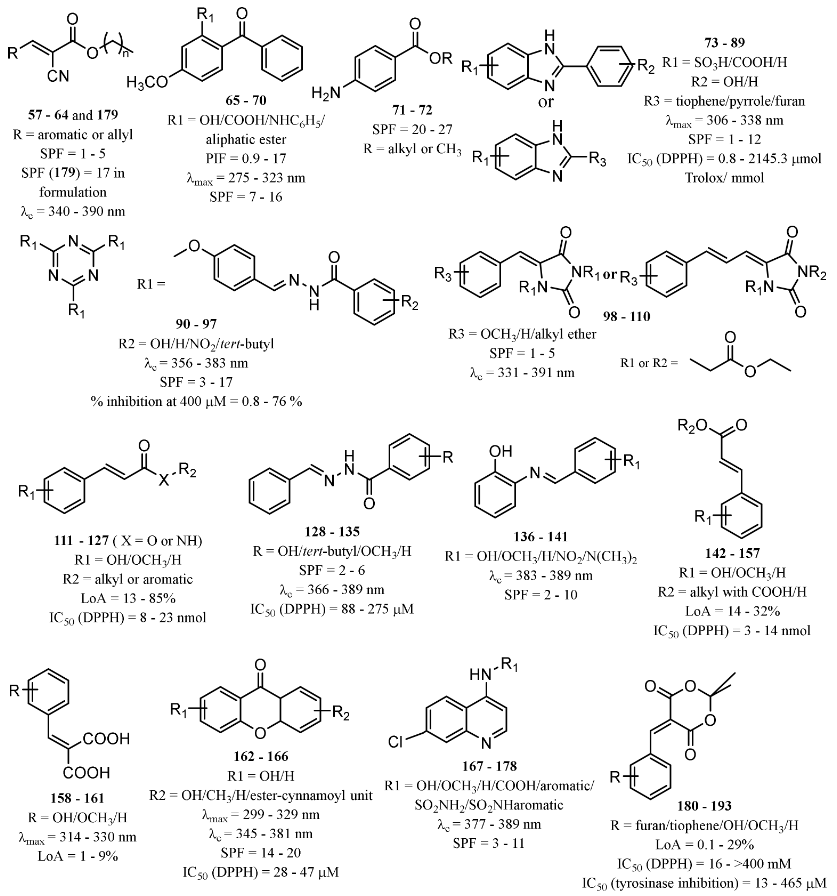
3.2.2.1. New Synthetic Derivatives Inspired by Commercialised UV Filters
3.2.2.2. Nature-Inspired Synthetised Compounds
3.2.2.3. Other New Synthetic Derivatives
4. Conclusions
References
- Jussila, A.; Huotari-Orava, R.; Ylianttila, L.; Partonen, T.; Snellman, E. Narrow-band ultraviolet B radiation induces the expression of beta-endorphin in human skin in vivo. J. Photochem. Photobiol. B 2016, 155, 104–108.
- Dahmane, R.; Pandel, R.; Trebse, P.; Poljsak, B. The role of sun exposure in skin aging. In Sun Exposure: Risk Factors, Protection Practices and Health Effects; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 1–40
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing Sunscreen Lifecycle to Minimize Environmental Risk Posed by Nanoparticulate UV-Filters—A Review for Safer-by-Design Products. Front. Environ. Sci. 2020, 8, 00101.
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049.
- Pawlowski, S.; Petersen-Thiery, M. Sustainable sunscreens: A challenge between performance, animal testing ban, and human and environmental safety. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, pp. 185–207.
- Abiola, T.T.; Whittock, A.L.; Stavros, V.G. Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy. Molecules 2020, 25, 3945.
- L’Oreal. Garnier Ambre Solaire. 2020. Available online: https://www.loreal.com/it-it/italy/press-release/group/garnier-ambre-solaire/ (accessed on 3 August 2021).
- Roelandts, R.; Vanhee, J.; Bonamie, A.; Kerkhofs, L.; Degreef, H. A Survey of Ultraviolet Absorbers in Commercially Available Sun Products. Int. J. Dermatol. 1983, 22, 247–255.
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council: Current Consolidated Version. Off. J. Eur. Union, (Last Current Consolidated Version Published on 1 October 2021); Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj (accessed on 26 December 2021).
- Bissonnette, R. Update on Sunscreens. Skin Ther. Lett. 2008. Available online: https://www.skintherapyletter.com/sunscreen/advances-update/ (accessed on 1 October 2021).
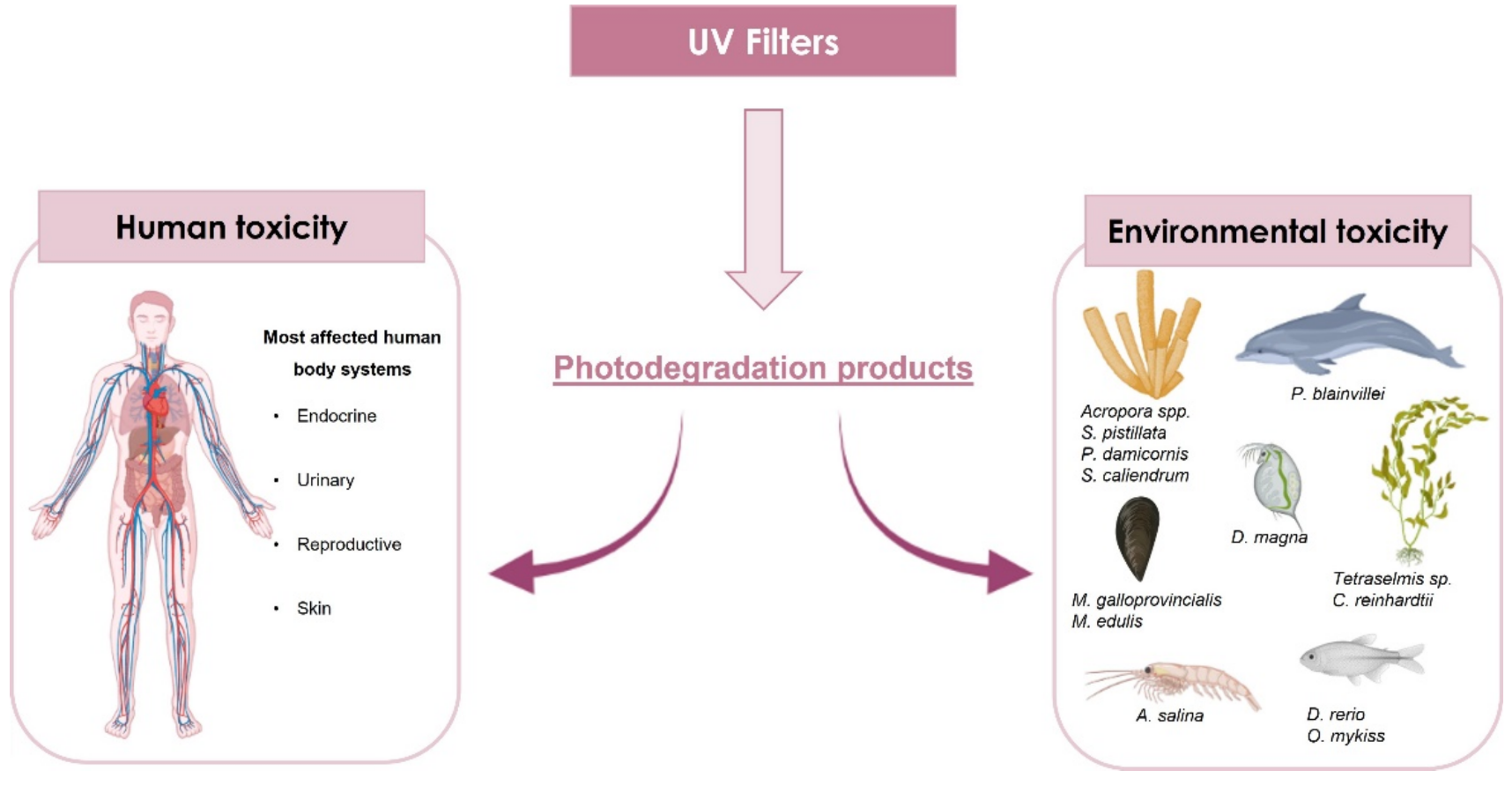
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Silvia Díaz-Cruz, M. Determination of parabens and benzophenone-type UV filters in human placenta: First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016, 88, 243–249.
- Rehfeld, A.; Egeberg, D.L.; Almstrup, K.; Petersen, J.H.; Dissing, S.; Skakkebæk, N.E. EDC IMPACT: Chemical UV filters can affect human sperm function in a progesterone-like manner. Endocr. Connect. 2018, 7, 16–25.
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279.
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161.
- Giraldo, A.; Montes, R.; Rodil, R.; Quintana, J.B.; Vidal-Liñán, L.; Beiras, R. Ecotoxicological Evaluation of the UV Filters Ethylhexyl Dimethyl p-Aminobenzoic Acid and Octocrylene Using Marine Organisms Isochrysis galbana, Mytilus galloprovincialis and Paracentrotus lividus. Arch. Environ. Contam. Toxicol. 2017, 72, 606–611.
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and toxicological effects of uv-filters on marine species. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, pp. 85–130.
- Bahia, M.F. Proteção Solar—Actualização, 1st ed.; Universidade do Porto: Porto, Portugal, 2003.
- Stiefel, C.; Schwack, W. Reactions of cosmetic UV filters with skin proteins: Model studies of esters with primary amines. Trends Photochem. Photobiol. 2013, 15, 105–116.
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110.
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353.
- Vega, J.; Bonomi-Barufi, J.; Gomez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659.
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537.
- Peyrot, C.; Mention, M.M.; Fournier, R.; Brunissen, F.; Couvreur, J.; Balaguer, P.; Allais, F. Expeditious and sustainable two-step synthesis of sinapoyl-l-malate and analogues: Towards non-endocrine disruptive bio-based and water-soluble bioactive compounds. Green Chem. 2020, 22, 6510–6518.
- Reis, J.S.; Corrêa, M.A.; Ribeiro, C.A.; Dos Santos, J.L. Synthesis and evaluation of 1,3,5-triazine derivatives as sunscreens useful to prevent skin cancer. Bioorg. Med. Chem. Lett. 2019, 29, 126755.
- Popiół, J.; Gunia-Krzyżak, A.; Słoczyńska, K.; Koczurkiewicz-Adamczyk, P.; Piska, K.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Krupa, A.; Żmudzki, P.; Marona, H.; et al. The involvement of xanthone and (E)-cinnamoyl chromophores for the design and synthesis of novel sunscreening agents. Int. J. Mol. Sci. 2021, 22, 34.
- Chen, L.L.; Tooley, I.; Wang, S.Q. Nanotechnology in photoprotection. In Nanotechnology in Dermatology; Springer: New York, NY, USA, 2013; pp. 229–236.
- Herzog, B.; Wehrle, M.; Quass, K. Photostability of UV absorber systems in sunscreens. Photochem. Photobiol. 2009, 85, 869–878.
- Bonda, C.A.; Lott, D. Sunscreen photostability. In Principles and Practice of Photoprotection; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 247–273.
- Afonso, S.; Horita, K.; Sousa E Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves Da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40.
- Duarte, J.; Almeida, I.F.; Costa, M.; Da Silva, E.S.; Faria, J.L.; Sousa Lobo, J.M.; Costa, P.C.; Scalia, S. Alginate microparticles as carriers for the UV filter 2-ethylhexyl 4-methoxycinnamate: Influence on photostability. Int. J. Cosmet. Sci. 2019, 41, 585–593.
- Chatelain, E.; Gabard, B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochem. Photobiol. 2001, 74, 401–406.
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139.
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability evaluation of five UV-filters, trans-resveratrol and beta-carotene in sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89.
- Paris, C.; Lhiaubet-Vallet, V.; Jimenez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184.
- De Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and efficacy evaluation of gelatin-based nanoparticles associated with UV filters. Colloids Surf. B Biointerfaces 2016, 140, 531–537.
- Graziola, F.; Candido, T.M.; De Oliveira, C.A.; Peres, D.D.; Issa, M.G.; Mota, J.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; et al. Gelatin-based microspheres crosslinked with glutaraldehyde and rutin oriented to cosmetics. Bras. J. Pharm. Sci. 2016, 52, 603–612.
- Juliano, C.; Magrini, G.A. Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 2017, 4, 11.
- Bordalo, D.; Leite, C.; Almeida, Â.; Soares, A.M.V.M.; Pretti, C.; Freitas, R. Impacts of UV filters in Mytilus galloprovincialis: Preliminary data on the acute effects induced by environmentally relevant concentrations. Sustainability 2020, 12, 6852.
- Picot Groz, M.; Martinez Bueno, M.J.; Rosain, D.; Fenet, H.; Casellas, C.; Pereira, C.; Maria, V.; Bebianno, M.J.; Gomez, E. Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC-MS/MS. Sci. Total Environ. 2014, 493, 162–169.
- Cunha, S.C.; Fernandes, J.O.; Vallecillos, L.; Cano-Sancho, G.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Maulvault, A.L.; Ferrari, F.; Fernandez-Tejedor, M.; et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots. Environ. Res. 2015, 143, 65–71.
- Shick, J.M.; Lesser, M.P.; Jokiel, P.L. Effects of ultraviolet radiation on corals and other coral reef organisms. Glob. Chang. Biol. 1996, 2, 527–545.
- Wijgerde, T.; van Ballegooijen, M.; Nijland, R.; van der Loos, L.; Kwadijk, C.; Osinga, R.; Murk, A.; Slijkerman, D. Adding insult to injury: Effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci. Total Environ. 2020, 733, 139030.
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285.
- Cahova, J.; Blahova, J.; Marsalek, P.; Doubkova, V.; Franc, A.; Garajová, M.; Tichy, F.; Mares, J.; Svobodova, Z. The biological activity of the organic UV filter ethylhexyl methoxycinnamate in rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2021, 774, 145570.
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Azevedo, A.; Lailson-Brito, J., Jr.; Torres, J.P.M.; et al. First determination of UV filters in marine mammals. octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625.
- Pico, Y.; Belenguer, V.; Corcellas, C.; Diaz-Cruz, M.S.; Eljarrat, E.; Farre, M.; Gago-Ferrero, P.; Huerta, B.; Navarro-Ortega, A.; Petrovic, M.; et al. Contaminants of emerging concern in freshwater fish from four Spanish Rivers. Sci. Total Environ. 2019, 659, 1186–1198.
- Duis, K.; Junker, T.; Coors, A. Review of the environmental fate and effects of two UV filter substances used in cosmetic products. Sci. Total Environ. 2022, 808, 151931.
- Krutmann, J.; Passeron, T.; Gilaberte, Y.; Granger, C.; Leone, G.; Narda, M.; Schalka, S.; Trullas, C.; Masson, P.; Lim, H.W. Photoprotection of the future: Challenges and opportunities. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 447–454.
- Yabe, S.; Sato, T. Cerium oxide for sunscreen cosmetics. J. Solid State Chem. 2003, 171, 7–11.
- Seixas, V.C.; Serra, O.A. Stability of sunscreens containing CePO4: Proposal for a new inorganic UV filter. Molecules 2014, 19, 9907–9925.
- Polonini, H.C.; Lopes, R.S.; Beatriz, A.; Gomes, R.S.; Silva, A.O.; De Lima, R.V.; Nunes, G.A.; Brandão, M.A.F.; Raposo, N.R.B.; De Lima, D.P. Synthesis and evaluation of octocrylene-inspired compounds for Uv-filter activity. Quim. Nova 2014, 37, 1004–1009.
- González, M.T.P.; Fumagalli, F.; Benevenuto, C.G.; da Silva Emery, F.; Gaspar, L.R. Novel benzophenone-3 derivatives with promising potential as UV filters: Relationship between structure, photoprotective potential and phototoxicity. Eur. J. Pharm. Sci. 2017, 101, 200–210.
- Gonçalves, M.C.; Rossoni, J.V., Jr.; Rabelo, A.C.S.; Costa, D.C.; Cazati, T.; Taylor, J.G.; Dos Santos, V.M.R. Preliminary studies of the cytotoxicity and photoprotective properties of benzophenone and lactone derivatives. Rev. Virtual Quim. 2018, 10, 600–608.
- Abbas, N.; Mahmood, Z.; Manzoor, S.; Akhtar, Z.; Tariq, M. Synthesis, characterization and sunscreen protection factor of novel synthesized Para Amino Benzoic Acid (PABA) esters. J. Chem. Soc. Pak. 2019, 41, 701–706.
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537.
- Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin damages—Structure activity relationship of benzimidazole derivatives bearing a 5-membered ring system. Molecules 2020, 25, 4324.
- Popiół, J.; Gunia-Krzyzak, A.; Piska, K.; Zelaszczyk, D.; Koczurkiewicz, P.; Słoczynska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Zesławska, E.; et al. Discovery of novel UV-filters with favorable safety profiles in the 5-arylideneimidazolidine-2,4-dione derivatives group. Molecules 2019, 24, 2321.
- Popiół, J.; Gunia-Krzyzak, A.; Piska, K.; Zelaszczyk, D.; Koczurkiewicz, P.; Słoczynska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Zesławska, E.; et al. Discovery of novel UV-filters with favorable safety profiles in the 5-arylideneimidazolidine-2,4-dione derivatives group. Molecules 2019, 24, 2321.
- Reis, J.S.; Corrêa, M.A.; Chung, M.C.; Dos Santos, J.L. Synthesis, antioxidant and photoprotection activities of hybrid derivatives useful to prevent skin cancer. Bioorg. Med. Chem. 2014, 22, 2733–2738.
- Horbury, M.D.; Holt, E.L.; Mouterde, L.M.M.; Balaguer, P.; Cebrian, J.; Blasco, L.; Allais, F.; Stavros, V.G. Towards symmetry driven and nature inspired UV filter design. Nat. Commun. 2019, 10, 4748.
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Balaguer, P.; Allais, F. Innovative bio-based organic UV-A and blue light filters from Meldrum’s acid. Molecules 2020, 25, 2178.
- Rioux, B.; Peyrot, C.; Mention, M.M.; Brunissen, F.; Allais, F. Sustainable synthesis of p-hydroxycinnamic diacids through proline-mediated knoevenagel condensation in Ethanol: An access to potent phenolic UV filters and radical scavengers. Antioxidants 2020, 9, 331.
- Resende, D.I.S.P.; Almeida, M.C.; Maciel, B.; Carmo, H.; Lobo, J.S.; Pozzo, C.D.; Cravo, S.M.; Rosa, G.P.; Kane-Pagès, A.; Do Carmo Barreto, M.; et al. Efficacy, stability, and safety evaluation of new polyphenolic xanthones towards identification of bioactive compounds to fight skin photoaging. Molecules 2020, 25, 2782.
- Giacomo, B.; Luca, B.; Nicola, L.; Luigi, R. Development of an innovative and eco-friendly UV radiation absorber, based on furan moieties. Cosmetics 2020, 7, 6.




