Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuang-Qing Zhang | + 1978 word(s) | 1978 | 2022-03-28 06:08:11 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1978 | 2022-03-29 09:07:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, S. Effects of Icaritin on Osteoporosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/21110 (accessed on 08 February 2026).
Zhang S. Effects of Icaritin on Osteoporosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/21110. Accessed February 08, 2026.
Zhang, Shuang-Qing. "Effects of Icaritin on Osteoporosis" Encyclopedia, https://encyclopedia.pub/entry/21110 (accessed February 08, 2026).
Zhang, S. (2022, March 28). Effects of Icaritin on Osteoporosis. In Encyclopedia. https://encyclopedia.pub/entry/21110
Zhang, Shuang-Qing. "Effects of Icaritin on Osteoporosis." Encyclopedia. Web. 28 March, 2022.
Copy Citation
Icaritin (ICT) is not only an enzyme-hydrolyzed product of icariin but also an intestinal metabolite of eight major flavonoids of the traditional Chinese medicinal plant Epimedium with extensive pharmacological activities, such as strengthening the kidney and reinforcing the bone. ICT displays several therapeutic effects, including osteoporosis prevention, neuroprotection, antitumor, cardiovascular protection, anti-inflammation, and immune-protective effect. ICT inhibits bone resorption activity of osteoclasts and stimulates osteogenic differentiation and maturation of bone marrow stromal progenitor cells and osteoblasts.
icaritin
antiosteoporosis
pharmacokineticcs
drug delivery systems
1. Introduction
Osteoporosis, a systemic skeletal disorder, is caused by excessive bone resorption over bone formation, characterized by decreased bone mineral density, microarchitectural deterioration, increased bone fragility and susceptibility to fracture [1][2]. Worldwide, over 200 million people are estimated to suffer from osteoporosis, in which women over the age of 50 or postmenopausal women are four times more likely to develop the disease than men [3]. Annually, osteoporosis causes more than 8.9 million fractures throughout the world, resulting in annual costs of more than 10 billion dollars [4].
Epimedium (Berberidaceae) is an important traditional Chinese medicinal plant and has long been used alone or in combination with other herbs for the treatment of various diseases, including osteoporosis, tendon health, cardiovascular diseases, sexual dysfunction, and menstrual irregularity [5]. There are more than 260 compounds identified from Epimedium including 141 flavonoids, 31 lignins, 12 ionones, 9 phenol glycosides, 6 phenylethanoid glycosides, 5 sesquiterpenes, and other types of moieties, of which flavonoids are the major components and important chemotaxonomic markers [6]. Icariin is the most abundant constituent and accounts for more than 5.0% of the dried weight of an alcoholic decoction of Epimedium [7]. Icaritin (ICT, Figure 1A) is not only a bioactive compound enzyme-hydrolyzed from icariin but also an intestinal metabolite of eight major flavonoids of Epimedium [8][9]. It exerts broad therapeutic capabilities such as osteoprotective effect [10], neuroprotective effect [11], cardiovascular protective effect [12], anti-cancer effect [13], anti-inflammation effect [14], and immune-protective effect [15]. Unfortunately, anhydroicaritin (Figure 1B) and wushanicaritin (Figure 1C) were regarded as ICT by mistake in some reports [6][16] possibly as they had similar chemical structures. On 10 January 2022, ICT was approved for the treatment of advanced hepatocellular carcinoma by China National Medical Products Administration. ICT is currently undergoing phase 1 clinical trial for the treatment of osteoporosis (ClinicalTrials.gov Identifier: NCT02931305). ICT targets osteogenesis pathways in mesenchymal stem cell, osteoblast, and osteoclast cell lineages, and displays beneficial effects on bone health in osteoporosis animal models [17]. Particularly, the prominent osteogenic effects of ICT made it a promising anti-osteoporotic drug candidate since ICT as a natural phytoestrogen may negate the high risks of hormone replacement therapy in clinic [8]. However, unfavorable intrinsic physicochemical and pharmacokinetic properties of ICT restrict its anti-osteoporotic effects, therefore, various novel drug delivery systems have been developed to dissolve the problems. Over the past decades, few literature reviews and book chapters have involved the topic. Therefore, osteogenic effects and mechanisms, pharmacokinetic properties and delivery systems of ICT are reviewed and discussed.
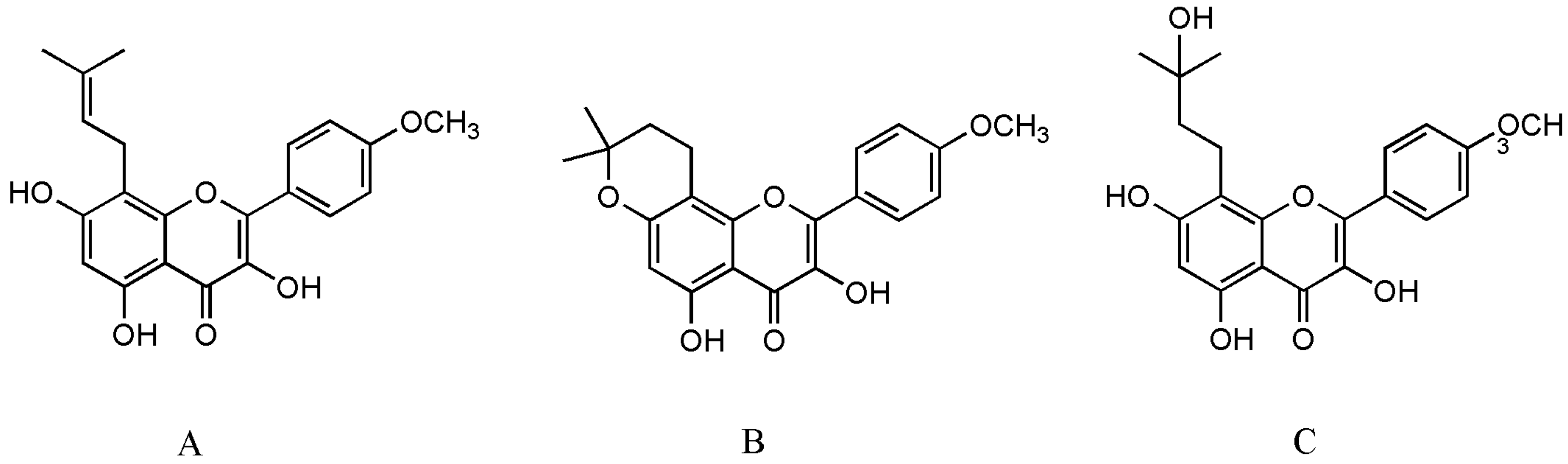
Figure 1. The structure of ICT (A), anhydroicaritin (B) and wushanicaritin (C).
2. Effects of Icaritin on Osteoporosis
Healthy bone, a dynamic living tissue, is mainly composed of two distinct types of cells: (1) osteoblasts, derived from bone marrow mesenchymal stromal cells (MSCs), which are bone-forming cells, and (2) osteoclasts, derived from bone marrow hematopoietic progenitors, which are multinucleated bone-resorbing cells. If the dynamic balance between bone formation and resorption is destroyed, bone metabolism disorders occur such as osteoporosis and osteopetrosis [18]. There are many factors that promote the occurrence and progression of osteoporosis, but the fundamental mechanism is an imbalance between osteoblasts and osteoclasts, including: (1) decreased differentiation and activity of osteoblasts result in reduced bone deposition, and (2) increased osteoclasts differentiation and activity lead to excessive bone resorption [19]. Therefore, osteoporosis therapy focuses on rebuilding the balance between bone formation and resorption.
2.1. Mesenchymal Stromal Cells
In 1951, Lorenz and his coworkers first verified that bone marrow acted as a pool of hematopoietic stem cells (HSCs) to maintain blood cells homeostasis for lifespan in mice and guinea pigs experiments [20]. Further research found that adult bone marrow-derived multipotent stem cells contained not only HSCs but also non-hematopoietic cells, that is MSCs. Then, MSCs have also been isolated from several other parts of the body including the adipose tissue, umbilical cord blood, skin, and amniotic fluid [21][22]. A series of studies, both in vivo and in vitro, have shown that MSCs have distinct differentiation potential under special microenvironment [23][24]. Since 1995, MSCs have been used in clinically studied experimental cell therapy for a wide range of diseases, such as systemic sclerosis [25], neurodegenerative diseases [26], liver regeneration [27][28], osteoarthritis [29], osteonecrosis [30], angiogenesis [31], ischemic brain [32], etc.
Fundamentally, MSCs are precursors for both osteoblasts and adipocytes, between which an inverse relationship exists (Figure 2). Bone loss is commonly accompanied by increasing bone marrow adiposity, that means MSCs play an important role in the development of osteoporosis. Therefore, promoting MSCs to osteogenesis is beneficial to bone regeneration. Several research have shown some small molecules such as ascorbic acid, β-glycerophosphate, and dexamethasone can stimulate osteoblastogenesis of MSCs by increasing the activity of alkaline phosphatase (ALP), calcium deposition, and suppressing adipogenesis of MSCs [33][34][35]. In 2013, Sheng et al. first reported that the novel semisynthetic molecule ICT could promote osteogenic differentiation and suppress adipogenesis of MSCs [10].
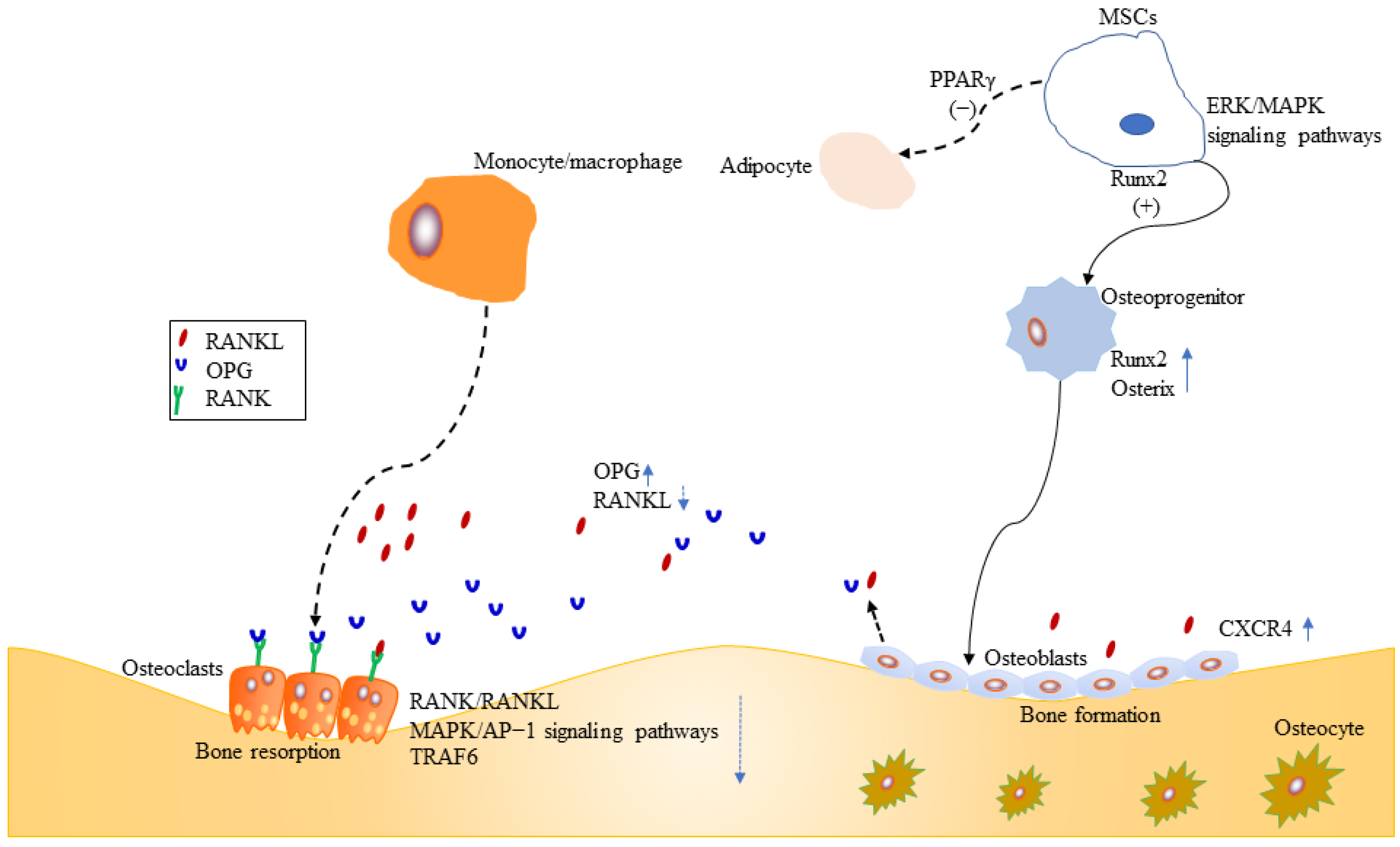
Figure 2. Effects of ICT on MSCs, osteoblasts and osteoclasts.
Runx2 is a key transcription factor for skeletal mineralization due to its regulation of extracellular matrix genes such as ALP, osteopontin, and type I collagen [36][37]. PPARγ plays an essential role in regulating development of the adipose lineage [38], and the relative activities of two transcription factors Runx2 and PPARγ determine whether MSCs differentiate into osteoblasts or adipocytes [39] (Figure 2). Sheng et al. [10] further identified that ICT promoted osteogenic differentiation and maturation of MSCs through initiating activation of Runx2. Meanwhile, their investigation [10] showed that ICT inhibited adipogenesis of MSCs through suppressing PPARγ (Figure 2).
Runx2 is activated by estrogen receptor and mitogen-activated protein kinase (ERK/MAPK)-dependent phosphorylation [39][40]. ERK/MAPK signaling pathways are essential for cellular biochemical and physiological processes including cell proliferation, migration, and differentiation. Luo et al. demonstrated that ICT could activate ERK/MAPK signaling pathway and facilitate the orientation of osteogenic differentiation of bone marrow MSCs in vitro [41] (Figure 2).
Stromal cell-derived factor-1 (SDF-1)/cysteine I-X-C motif chemokine receptor 4 (CXCR4) axis is required for mobilization and recruitment of MSCs, as well as proliferation and survival of MSCs [42][43] (Figure 2). Using an in vitro cell culture model, Lim et al. demonstrated that ICT enhanced MSCs proliferation, chemotaxis to SDF-1, and osteogenic differentiation, through the activation of signal transduction activator transcription factor (STAT-3) [44].
2.2. Osteoblasts
Osteoblasts, originating from MSCs, mediate bone-formation of new bone tissue, which is coupled by osteoclast-mediated bone resorption of old bone tissue. Therefore, stimulation of osteoblasts proliferation and activation is a target for new bone-forming to prevent osteoporosis. Osteoprogenitors, osteoblasts, and osteocytes belong to osteoblast lineage cells. The mature osteoblasts, located on the bone surfaces, are responsible for the synthesis and mineralization of the organic matrix rich in type I collagen and osteocalcin in the initial bone formation phase [45][46]. Early osteoprogenitors express Runx2 and osterix which are two critical transcription factors for osteoblasts differentiation and function [45][47][48][49][50] (Figure 2). CXCR4 is critical for maintaining osteoblast anabolic function. ICT enhanced the differentiation of MC3T3-E1 preosteoblastic cells and subsequently resulted in mineralization, collagen synthesis, and bone formation, because ICT promoted mRNA and protein expression of bone-forming biomarkers, such as ALP, type I collagen, osteocalcin, OPN, and Runx2 [51]. Further study revealed the mechanisms might be associated with ERK/MAP signaling pathway activated by ICT [51]. Wei et al. found that ICT could promote maturation and mineralization of MC3T3-E1 cells through SDF-1/CXCR4 signaling pathway in a series of in vitro experiments [52]. Lim et al. examined whether ICT could increase human osteoblast anabolic function. Both cellular and animal experiments showed that ICT increased osteoblasts proliferation and function. The underlying mechanism was that ICT suppressed the phosphorylation of STAT-3 to upregulate CXCR4 expression [53]. Peng et al. performed a study to examine the relationship between ICT treatment initiation time and bone turnover markers in adult ovariectomized rats, and found that early ICT treatment (1 month post-operation), not late ICT treatment (3 months post-operation), exerted beneficial effects on osteoporotic bone in ovariectomized rats [54]. Meanwhile, they performed a series of experiments to evaluate the population of osteoblasts with colony formation assays, assess the expression levels of osteoblasts-related genes by real-time polymerase chain reaction. Late ICT treatment failed to increase bone-forming related parameters [54].
Osteoblasts play an important role in maintaining bone homeostasis. Mature osteoblasts produce type I collagen and osteocalcin, and regulate activity of osteoclasts through receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) [45] (Figure 2). Currently, OPG/RANKL/RANK signaling pathway is a crucial signal pathway for bone remodeling for the interaction between osteoblasts and osteoclasts, as well as a major pathway for affecting bone metabolism and for preventing and treating osteoporosis [55][56][57]. The trimolecular complex belongs to the superfamily of tumor necrosis factor (TNF) [58]. The receptor activator of NF-κB (RANK) is located on the osteoclasts surface, and its major ligands are OPG and RANKL. OPG, a secreted glycoprotein, has been identified to regulate bone resorption [57][59]. RANKL is mainly derived from osteocytes, osteoblasts, and MSCs during bone remodeling [58], and RANK is the only known receptor for RANKL. OPG and RANKL compete for RANK, which determines the balance between bone remodeling and resorption [60]. Huang et al. demonstrated that Epimedium-derived prenylflavonoids including icariin, icariside II, and ICT could promote proliferation, alkaline phosphatase activity, osteocalcin secretion of osteoblasts, and matrix mineralization [61]. Furthermore, the experiment demonstrated that ICT was more potent than other extracts, because ICT increased mRNA expression of OPG, and inhibited mRNA expression of RANKL [61].
2.3. Osteoclasts
Osteoclasts, arose from bone marrow hematopoietic monocyte/macrophage progenitors, mediate bone resorption of old bone tissue. The regulation of osteoclasts differentiation and activation involve signaling induced by RANKL and its receptor RANK [62][63][64][65][66][67] (Figure 2). Once RANK is activated by RANKL, osteoclasts differentiate and promote bone resorption. However, excessive activation of RANK/RANKL signaling pathway leads to osteoporosis. Therefore, inhibition of the RANKL-induced osteoclasts formation is an effective therapy for osteoporosis. TNF receptor-associated factor6 (TRAF6), a critical adaptor protein, is necessary for bone resorption [68], which is supported by several studies that TRAF6-deficient mice generated osteopetrosis [69][70][71]. Liu et al. evaluated the growth inhibitory effect of ICT on preosteoclastic RAW264.7 cells, and found ICT suppressed osteoclastic differentiation and activity in a dose-dependent manner [72]. Furthermore, Tan et al. revealed that ICT suppressed osteoclastogenesis in two osteoclast precursor models, RAW 264.7 mouse monocyte cell line and human PBMC, through inhibition of RANK/RANKL and MAPK/AP-1 signaling pathways and promotion of proteasomal degradation of TRAF6 [73]. A randomized, double-blind, placebo-controlled trial showed that ICT increased the bone anabolism marker such as bone specific alkaline phosphatase and suppressed TRAF6 protein in peripheral blood osteoclast-precursor monocytes in post-menopausal women [74].
2.4. Inflammation and Osteoporosis
Recently, more attention has been paid on the relationship between inflammation and osteoporosis. Evidence has shown that inflammatory cytokines play an important role in the osteoporosis processing of hormonal deficiency-induced rat model [75][76]. Accumulating data suggested that pro-inflammatory cytokines such as interleukins (IL), tumor necrosis factor-alpha (TNF-α), chemokines, interferons could induce osteoclastic bone resorption [77][78]. In 2013, Lai et al. first demonstrated the anti-inflammatory effect of ICT in lipopolysaccharide (LPS)-induced mouse peritoneal macrophages in vitro and peritonitis model in vivo [14]. In this report, the researchers found pretreatment of ICT significantly could inhibit the inflammatory cytokines production, including IL-6, IL-10, MCP-1, IFN, TNF, and IL-12p70 [14]. Then anti-inflammatory activity of icariin and its metabolites have been widely reported in different areas of disease [79][80][81].
2.5. Animal Bone Defect Model
Based on the findings that ICT might be a therapeutic small molecule agent for bone reconstruction, a series of animal bone defect model experiments systematically evaluated bone regeneration of bioactive scaffolds incorporating phytomolecule ICT which served as exogenous growth factor [82][83][84]. Not only in the non-loading bearing mechanical stresses rat calvarial defects model, but also in a standard rabbit ulnar segmental defect model, the bioactive scaffold incorporating ICT enhanced newly mineralized bone and new vessel growth. In conclusion, as an exogenous growth factor, ICT is beneficial to bone regeneration.
References
- Ordikhani, F.; Zandi, N.; Mazaheri, M.; Luther, G.A.; Ghovvati, M.; Akbarzadeh, A.; Annabi, N. Targeted nanomedicines for the treatment of bone disease and regeneration. Med. Res. Rev. 2021, 41, 1221–1254.
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone diseases: Current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials 2020, 10, 875.
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9.
- Li, N.; Cornelissen, D.; Silverman, S.; Pinto, D.; Si, L.; Kremer, I.; Bours, S.; de Bot, R.; Boonen, A.; Evers, S.; et al. An Updated Systematic Review of Cost-Effectiveness Analyses of Drugs for Osteoporosis. Pharmacoeconomics 2021, 39, 181–209.
- Zhang, S.Q. Biodistribution evaluation of icaritin in rats by ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2014, 155, 1382–1387.
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541.
- Comission, C.P. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science Press: Beijing, China, 2020; Volume I.
- Zhang, S.Q. Ultra-high performance liquid chromatography-tandem mass spectrometry for the quantification of icaritin in mouse bone. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 978–979, 24–28.
- Zhang, S.Q.; Zhang, S.Z. Oral absorption, distribution, metabolism, and excretion of icaritin in rats by Q-TOF and UHPLC-MS/MS. Drug Test. Anal. 2017, 9, 1604–1610.
- Sheng, H.; Rui, X.F.; Sheng, C.J.; Li, W.J.; Cheng, X.Y.; Jhummon, N.P.; Yu, Y.C.; Qu, S.; Zhang, G.; Qin, L. A novel semisynthetic molecule icaritin stimulates osteogenic differentiation and inhibits adipogenesis of mesenchymal stem cells. Int. J. Med. Sci. 2013, 10, 782–789.
- Wang, Z.; Zhang, X.; Wang, H.; Qi, L.; Lou, Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007, 145, 911–922.
- Wo, Y.B.; Zhu, D.Y.; Hu, Y.; Wang, Z.Q.; Liu, J.; Lou, Y.J. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J. Cell. Biochem. 2008, 103, 1536–1550.
- Tao, C.C.; Wu, Y.; Gao, X.; Qiao, L.; Yang, Y.; Li, F.; Zou, J.; Wang, Y.H.; Zhang, S.Y.; Li, C.L.; et al. The antitumor effects of icaritin against breast cancer is related to estrogen receptors. Curr. Mol. Med. 2020, 21, 73–85.
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49.
- Qin, S.K.; Li, Q.; Ming Xu, J.; Liang, J.; Cheng, Y.; Fan, Y.; Jiang, J.; Ye, H.; Tao, H.; Li, L.; et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival. Cancer Sci. 2020, 111, 4218–4231.
- Zheng, Z.G.; Zhang, X.; Zhou, Y.P.; Lu, C.; Thu, P.M.; Qian, C.; Zhang, M.; Li, P.; Li, H.J.; Xu, X. Anhydroicaritin, a SREBPs inhibitor, inhibits RANKL-induced osteoclastic differentiation and improves diabetic osteoporosis in STZ-induced mice. Eur. J. Pharmacol. 2017, 809, 156–162.
- Indran, I.R.; Liang, R.L.; Min, T.E.; Yong, E.L. Preclinical studies and clinical evaluation of compounds from the genus Epimedium for osteoporosis and bone health. Pharmacol. Ther. 2016, 162, 188–205.
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012, 2012, 148261.
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in bone erosive diseases: Perspectives in osteoporosis treatment. Trends Endocrinol. Metab. 2021, 32, 76–94.
- Tao, H.; Ma, D.D. Evidence for transdifferentiation of human bone marrow-derived stem cells: Recent progress and controversies. Pathology 2003, 35, 6–13.
- Ugurlu, B.; Karaoz, E. Comparison of similar cells: Mesenchymal stromal cells and fibroblasts. Acta Histochem. 2020, 122, 151634.
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12.
- Pino, A.M.; Rosen, C.J.; Rodriguez, J.P. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol. Res. 2012, 45, 279–287.
- Hardy, R.; Cooper, M.S. Glucocorticoid-induced osteoporosis-a disorder of mesenchymal stromal cells? Front. Endocrinol. 2011, 2, 24.
- Farge, D.; Loisel, S.; Lansiaux, P.; Tarte, K. Mesenchymal stromal cells for systemic sclerosis treatment. Autoimmun. Rev. 2021, 20, 102755.
- Teli, P.; Kale, V.; Vaidya, A. Extracellular vesicles isolated from mesenchymal stromal cells primed with neurotrophic factors and signaling modifiers as potential therapeutics for neurodegenerative diseases. Curr. Res. Transl. Med. 2021, 69, 103286.
- Hu, C.; Wu, Z.; Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 2020, 16, 893–903.
- Wang, L.; Li, S.; Wang, H.Y.; Zeng, J.; Zhang, Z.Z.; Lv, D.Y.; Kuang, W.H. In a rat model of acute liver failure, icaritin improved the therapeutic effect of mesenchymal stem cells by activation of the hepatocyte growth factor/c-Met pathway. Evid. Based Complement. Alternat. Med. 2019, 2019, 4253846.
- Ruiz, M.; Toupet, K.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noel, D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials 2020, 226, 119544.
- Elgaz, S.; Bonig, H.; Bader, P. Mesenchymal stromal cells for osteonecrosis. J. Transl. Med. 2020, 18, 399.
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol. Life Sci. 2020, 77, 253–265.
- Borlongan, C.V.; Glover, L.E.; Tajiri, N.; Kaneko, Y.; Freeman, T.B. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol. 2011, 95, 213–228.
- Yamada, A.; Iwata, T.; Yamato, M.; Okano, T.; Izumi, Y. Diverse functions of secreted frizzled-related proteins in the osteoblastogenesis of human multipotent mesenchymal stromal cells. Biomaterials 2013, 34, 3270–3278.
- Baker, N.; Sohn, J.; Tuan, R.S. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res. Ther. 2015, 6, 238.
- Iwata, T.; Kawamoto, T.; Sasabe, E.; Miyazaki, K.; Fujimoto, K.; Noshiro, M.; Kurihara, H.; Kato, Y. Effects of overexpression of basic helix-loop-helix transcription factor Dec1 on osteogenic and adipogenic differentiation of mesenchymal stem cells. Eur. J. Cell Biol. 2006, 85, 423–431.
- Westendorf, J.J. Transcriptional co-repressors of Runx2. J. Cell. Biochem. 2006, 98, 54–64.
- Bae, J.S.; Gutierrez, S.; Narla, R.; Pratap, J.; Devados, R.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Javed, A. Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation. J. Cell. Biochem. 2007, 100, 434–449.
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156.
- Ge, C.; Cawthorn, W.P.; Li, Y.; Zhao, G.; Macdougald, O.A.; Franceschi, R.T. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J. Cell. Physiol. 2016, 231, 587–596.
- Li, Y.; Ge, C.; Franceschi, R.T. MAP Kinase-dependent RUNX2 phosphorylation is necessary for epigenetic modification of chromatin during osteoblast differentiation. J. Cell. Physiol. 2017, 232, 2427–2435.
- Luo, G.; Xu, B.; Wang, W.; Wu, Y.; Li, M. Study of the osteogenesis effect of icariside II and icaritin on canine bone marrow mesenchymal stem cells. J. Bone Miner. Metab. 2018, 36, 668–678.
- Herberg, S.; Kondrikova, G.; Hussein, K.A.; Johnson, M.H.; Elsalanty, M.E.; Shi, X.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Mesenchymal stem cell expression of stromal cell-derived factor-1beta augments bone formation in a model of local regenerative therapy. J. Orthop. Res. 2015, 33, 174–184.
- Herberg, S.; Shi, X.; Johnson, M.H.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS ONE 2013, 8, e58207.
- Lim, R.Z.L.; Li, L.; Yong, E.L.; Chew, N. STAT-3 regulation of CXCR4 is necessary for the prenylflavonoid Icaritin to enhance mesenchymal stem cell proliferation, migration and osteogenic differentiation. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1680–1692.
- Dirckx, N.; Van Hul, M.; Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 170–191.
- He, L.; Lee, J.; Jang, J.H.; Sakchaisri, K.; Hwang, J.; Cha-Molstad, H.J.; Kim, K.A.; Ryoo, I.J.; Lee, H.G.; Kim, S.O.; et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell Signal. 2013, 25, 552–560.
- Adhami, M.D.; Rashid, H.; Chen, H.; Clarke, J.C.; Yang, Y.; Javed, A. Loss of Runx2 in committed osteoblasts impairs postnatal skeletogenesis. J. Bone Miner. Res. 2015, 30, 71–82.
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44 (Suppl. 1), 109–116.
- Pratap, J.; Galindo, M.; Zaidi, S.K.; Vradii, D.; Bhat, B.M.; Robinson, J.A.; Choi, J.Y.; Komori, T.; Stein, J.L.; Lian, J.B.; et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003, 63, 5357–5362.
- Artigas, N.; Urena, C.; Rodriguez-Carballo, E.; Rosa, J.L.; Ventura, F. Mitogen-activated protein kinase (MAPK)-regulated interactions between Osterix and Runx2 are critical for the transcriptional osteogenic program. J. Biol. Chem. 2014, 289, 27105–27117.
- Wu, Z.; Ou, L.; Wang, C.; Yang, L.; Wang, P.; Liu, H.; Xiong, Y.; Sun, K.; Zhang, R.; Zhu, X. Icaritin induces MC3T3-E1 subclone14 cell differentiation through estrogen receptor-mediated ERK1/2 and p38 signaling activation. Biomed. Pharmacother. 2017, 94, 1–9.
- Wei, Z.; Shi, W.; Chen, K.; Zhou, J.; Wang, M. Icaritin promotes maturation and mineralization of mouse osteoblast MC3T3-E1 cells through CXCR4/SDF-1 signal pathway. Zhejiang Da Xue Xue Bao Yi Xue Ban 2017, 46, 571–577.
- Lim, R.Z.L.; Li, L.; Chew, N.; Yong, E.L. The prenylflavonoid Icaritin enhances osteoblast proliferation and function by signal transducer and activator of transcription factor 3 (STAT-3) regulation of C-X-C chemokine receptor type 4 (CXCR4) expression. Bone 2017, 105, 122–133.
- Peng, S.; Zhang, G.; Zhang, B.-T.; Guo, B.; He, Y.; Bakker, A.J.; Pan, X.; Zhen, W.; Hung, L.; Qin, L.; et al. The beneficial effect of Icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone 2013, 55, 230–240.
- Kohli, S.S.; Kohli, V.S. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181.
- Pivonka, P.; Zimak, J.; Smith, D.W.; Gardiner, B.S.; Dunstan, C.R.; Sims, N.A.; Martin, T.J.; Mundy, G.R. Theoretical investigation of the role of the RANK-RANKL-OPG system in bone remodeling. J. Theor. Biol. 2010, 262, 306–316.
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319.
- Fouque-Aubert, A.; Chapurlat, R. Influence of RANKL inhibition on immune system in the treatment of bone diseases. Jt. Bone Spine 2008, 75, 5–10.
- Wang, Z.; Ding, L.; Zhang, S.; Jiang, T.; Yang, Y.; Li, R. Effects of icariin on the regulation of the OPG-RANKL-RANK system are mediated through the MAPK pathways in IL-1beta-stimulated human SW1353 chondrosarcoma cells. Int. J. Mol. Med. 2014, 34, 1720–1726.
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192.
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840.
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545.
- Wang, Y.; Hou, J.F.; Zhou, Z.L. Chicken receptor activator of nuclear factor-kappaB ligand induces formation of chicken osteoclasts from bone marrow cells and also directly activates mature osteoclasts. Poult. Sci. 2008, 87, 2344–2349.
- Mangashetti, L.S.; Khapli, S.M.; Wani, M.R. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-kappa B and Ca2+ signaling. J. Immunol. 2005, 175, 917–925.
- Moreno, J.L.; Kaczmarek, M.; Keegan, A.D.; Tondravi, M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood 2003, 102, 1078–1086.
- Hakeda, Y.; Kobayashi, Y.; Yamaguchi, K.; Yasuda, H.; Tsuda, E.; Higashio, K.; Miyata, T.; Kumegawa, M. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem. Biophys. Res. Commun. 1998, 251, 796–801.
- Burgess, T.L.; Qian, Y.; Kaufman, S.; Ring, B.D.; Van, G.; Capparelli, C.; Kelley, M.; Hsu, H.; Boyle, W.J.; Dunstan, C.R.; et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell. Biol. 1999, 145, 527–538.
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280.
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024.
- Naito, A.; Azuma, S.; Tanaka, S.; Miyazaki, T.; Takaki, S.; Takatsu, K.; Nakao, K.; Nakamura, K.; Katsuki, M.; Yamamoto, T.; et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 1999, 4, 353–362.
- Weisz, H.M.; Basel-Vanagaite, L.; Krauss, A.; Konen, O.; Levy, Y.; Garty, B.Z.; Smirin-Yosef, P.; Maya, I.; Lagovsky, I.; Taub, E.; et al. Homozygous deletion of RAG1, RAG2 and 5′ region TRAF6 causes severe immune suppression and atypical osteopetrosis. Clin. Genet. 2017, 91, 902–907.
- Liu, Y.-Q.; Yang, Q.-X.; Cheng, M.-C.; Xiao, H.-B. Synergistic inhibitory effect of Icariside II with Icaritin from Herba Epimedii on pre-osteoclastic RAW264.7 cell growth. Phytomedicine 2014, 21, 1633–1637.
- Tan, E.M.; Li, L.; Indran, I.R.; Chew, N.; Yong, E.L. TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J. Bone Miner. Res. 2017, 32, 846–860.
- Yong, E.L.; Cheong, W.F.; Huang, Z.; Thu, W.P.P.; Cazenave-Gassiot, A.; Seng, K.Y.; Logan, S. Randomized, double-blind, placebo-controlled trial to examine the safety, pharmacokinetics and effects of Epimedium prenylflavonoids, on bone specific alkaline phosphatase and the osteoclast adaptor protein TRAF6 in post-menopausal women. Phytomedicine 2021, 91, 153680.
- Xu, B.; He, Y.; Lu, Y.; Ren, W.; Shen, J.; Wu, K.; Xu, K.; Wu, J.; Hu, Y. Glucagon like peptide 2 has a positive impact on osteoporosis in ovariectomized rats. Life Sci. 2019, 226, 47–56.
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075.
- Mundy, G.R. Osteoporosis and inflammation. Nutr. Rev. 2007, 65, S147–S151.
- Briot, K.; Geusens, P.; Em, B.I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314.
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and its metabolites as potential protective phytochemicals against Alzheimer’s disease. Front. Pharmacol. 2019, 10, 271.
- Hwang, E.; Lin, P.; Ngo, H.T.T.; Gao, W.; Wang, Y.S.; Yu, H.S.; Yi, T.H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-kappaB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408.
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015, 126, 57–68.
- Chen, S.H.; Lei, M.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, X.L.; Li, W.; Zhao, Z.; Kong, A.; Xiao, D.M.; et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013, 9, 6711–6722.
- Chen, S.H.; Zheng, L.-Z.; Xie, X.H.; Wang, X.L.; Lai, Y.X.; Chen, S.K.; Zhang, M.; Wang, Y.X.; Griffith, J.F.; Qin, L. Comparative study of poly (lactic-co-glycolic acid)/tricalcium phosphate scaffolds incorporated or coated with osteogenic growth factors for enhancement of bone regeneration. J. Orthop. Transl. 2014, 2, 91–104.
- Shi, G.S.; Li, Y.Y.; Luo, Y.P.; Jin, J.F.; Sun, Y.X.; Zheng, L.Z.; Lai, Y.X.; Li, L.; Fu, G.H.; Qin, L.; et al. Bioactive PLGA/tricalcium phosphate scaffolds incorporating phytomolecule icaritin developed for calvarial defect repair in rat model. J. Orthop. Transl. 2020, 24, 112–120.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
846
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




