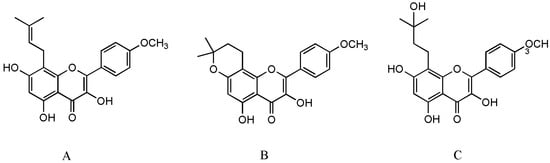
Osteoporosis, a systemic skeletal disorder, is caused by excessive bone resorption over bone formation, characterized by decreased bone mineral density, microarchitectural deterioration, increased bone fragility and susceptibility to fracture [
1,
2]. Worldwide, over 200 million people are estimated to suffer from osteoporosis, in which women over the age of 50 or postmenopausal women are four times more likely to develop the disease than men [
3]. Annually, osteoporosis causes more than 8.9 million fractures throughout the world, resulting in annual costs of more than 10 billion dollars [
4].
Epimedium (Berberidaceae) is an important traditional Chinese medicinal plant and has long been used alone or in combination with other herbs for the treatment of various diseases, including osteoporosis, tendon health, cardiovascular diseases, sexual dysfunction, and menstrual irregularity [
5]. There are more than 260 compounds identified from
Epimedium including 141 flavonoids, 31 lignins, 12 ionones, 9 phenol glycosides, 6 phenylethanoid glycosides, 5 sesquiterpenes, and other types of moieties, of which flavonoids are the major components and important chemotaxonomic markers [
6]. Icariin is the most abundant constituent and accounts for more than 5.0% of the dried weight of an alcoholic decoction of
Epimedium [
7]. Icaritin (ICT,
Figure 1A) is not only a bioactive compound enzyme-hydrolyzed from icariin but also an intestinal metabolite of eight major flavonoids of
Epimedium [
8,
9]. It exerts broad therapeutic capabilities such as osteoprotective effect [
10], neuroprotective effect [
11], cardiovascular protective effect [
12], anti-cancer effect [
13], anti-inflammation effect [
14], and immune-protective effect [
15]. Unfortunately, anhydroicaritin (
Figure 1B) and wushanicaritin (
Figure 1C) were regarded as ICT by mistake in some reports [
6,
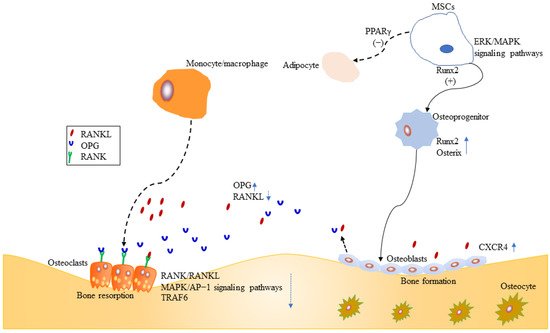
16] possibly as they had similar chemical structures. On 10 January 2022, ICT was approved for the treatment of advanced hepatocellular carcinoma by China National Medical Products Administration. ICT is currently undergoing phase 1 clinical trial for the treatment of osteoporosis (ClinicalTrials.gov Identifier: NCT02931305). ICT targets osteogenesis pathways in mesenchymal stem cell, osteoblast, and osteoclast cell lineages, and displays beneficial effects on bone health in osteoporosis animal models [
17]. Particularly, the prominent osteogenic effects of ICT made it a promising anti-osteoporotic drug candidate since ICT as a natural phytoestrogen may negate the high risks of hormone replacement therapy in clinic [
8]. However, unfavorable intrinsic physicochemical and pharmacokinetic properties of ICT restrict its anti-osteoporotic effects, therefore, various novel drug delivery systems have been developed to dissolve the problems. Over the past decades, few literature reviews and book chapters have involved the topic. Therefore, osteogenic effects and mechanisms, pharmacokinetic properties and delivery systems of ICT are reviewed and discussed.