Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hesam Kamyab | + 2504 word(s) | 2504 | 2022-02-16 04:32:53 | | | |
| 2 | Jason Zhu | -16 word(s) | 2488 | 2022-03-28 03:51:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kamyab, H. Electrocoagulation Process on Agro-Based Industrial Wastewater. Encyclopedia. Available online: https://encyclopedia.pub/entry/21100 (accessed on 07 February 2026).
Kamyab H. Electrocoagulation Process on Agro-Based Industrial Wastewater. Encyclopedia. Available at: https://encyclopedia.pub/entry/21100. Accessed February 07, 2026.
Kamyab, Hesam. "Electrocoagulation Process on Agro-Based Industrial Wastewater" Encyclopedia, https://encyclopedia.pub/entry/21100 (accessed February 07, 2026).
Kamyab, H. (2022, March 28). Electrocoagulation Process on Agro-Based Industrial Wastewater. In Encyclopedia. https://encyclopedia.pub/entry/21100
Kamyab, Hesam. "Electrocoagulation Process on Agro-Based Industrial Wastewater." Encyclopedia. Web. 28 March, 2022.
Copy Citation
The electrocoagulation process can be used for agro-based wastewater treatment. The performance of the electrocoagulation process is based on several parameters, including the electrode materials, electrolysis time, current density, and electrolyte support. Agro-based industrial wastewater (AIW) treatment processes depend on the characteristics of the wastewater. The removal of organic content from various sources of AIW can reach up to more than 80%.
Wastewater
electrocoagulation
Coagulation
1. Fundamental
1.1. Coagulation Principal
Coagulation is a traditional physicochemical treatment using phase separation for the pollutants of wastewaters before they are released to the environment [1]. The addition of inorganic or organic compounds to the coagulation process destabilizes the particles, affecting the electrical double layer (EDL). The impact to the environment from the coagulation process in the generation of sludge makes the concern more serious in terms of management, treatment and operational cost [2]. The sludge also contains residual metal ions from the coagulation process [3].
Chemicals known as coagulants lower the energy barrier between particles. As a result of the weak bond, the particles can agglomerate more easily [4]. Aluminium and iron metal salts are common coagulants used in wastewater treatment. Both metals can produce multivalent ions such as Al3+, Fe2+, and Fe3+, as well as a variety of hydrolysis products. Because it oxidizes to Fe (III) during the coagulation process to increase efficiency, Fe (II) is a poor coagulant [5]. Simple aluminium, iron sulfates, and chlorides are the most commonly used salts [6]. The non-hydrolyzed metal coagulants include Al2(SO4)3, FeSO4, AlCl3 and FeCl3 [7]. Meanwhile, there is another metal coagulant that comes with several advantages, which is named pre-hydrolyzed metal coagulant [8]. Examples of pre-hydrolyzed metal coagulants are polyaluminum chloride and sulfates [9]. The pre-hydrolyzed metal coagulants have advantages such as being more effective than non-hydrolyzed metal coagulants and less sensitive to changes in pH and temperature [10].
1.2. Electrocoagulation
Electrocoagulation by using metal hydroxide has a high ability to adsorb pollutants [11]. The electrode materials commonly use Al and Fe. When the electricity is supplied into the electrode, the electrode will generate ions (Fe2+, Fe3+, and Al3+) and produce the coagulant. The electrocoagulation reaction is usually followed by an electro-flotation reaction [12]. The aluminium anode generates cationic forms such as Al3+ and Al(OH)2+ in acidic conditions. However, in alkaline conditions, these species are transformed into Al (OH)3, dimer compounds such as Al2(O)(OH)4 and Al2(OH)24+. They can also can be transformed into a more complex compound [13].
Figure 1 shows the schematic diagram of electrocoagulation. Several reactions happen during electrocoagulation for the production of hydroxides. For the electrode materials using aluminium, the oxidation takes place at the anode, as follows:
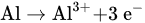
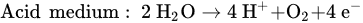
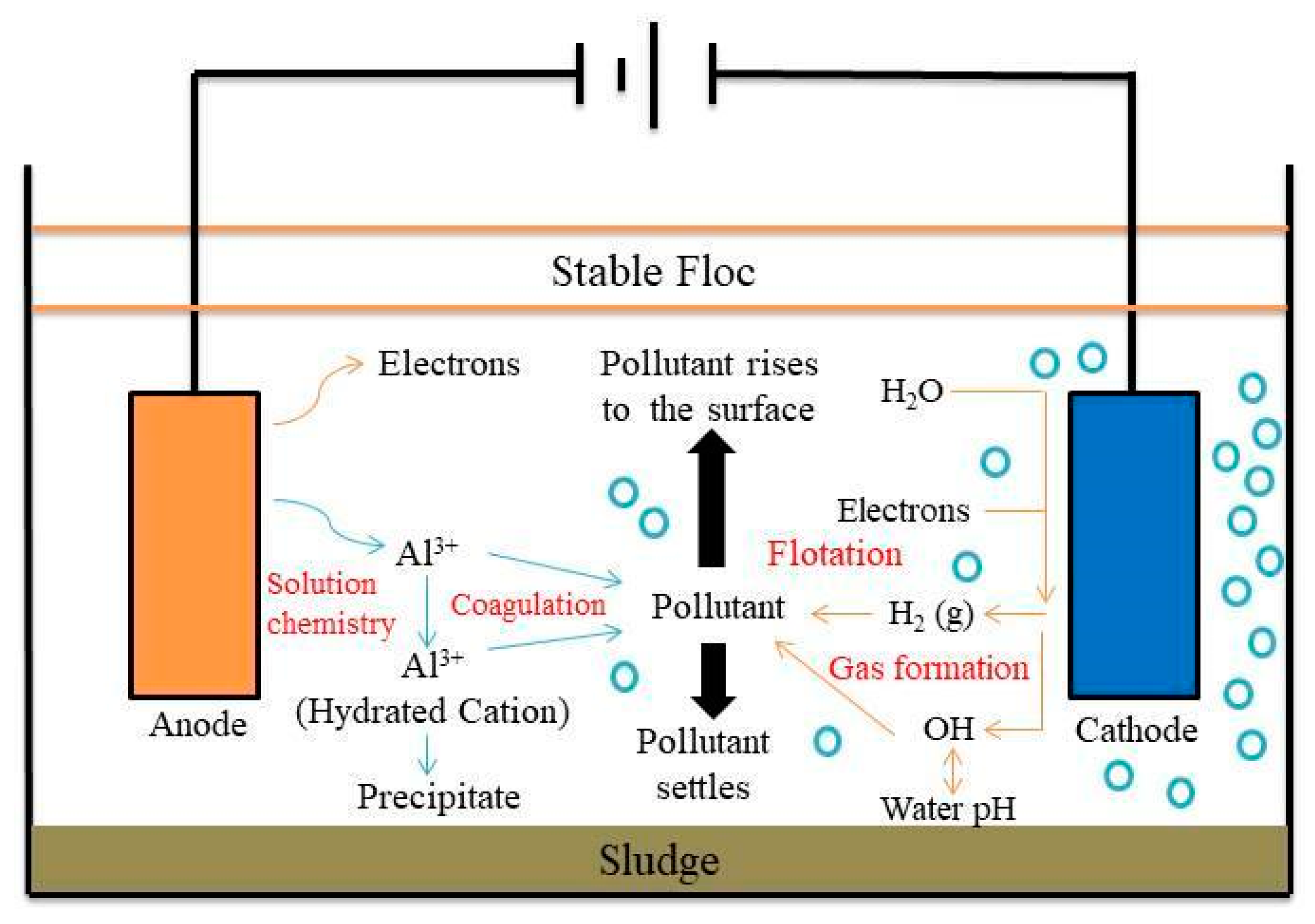
Figure 1. Schematic diagram of the electrocoagulation process.
In addition, a secondary reaction possibly occurs during the oxidation process. The formation of oxygen by the electrolysis of water is divided into two conditions: acidic and basic media.
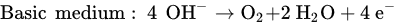
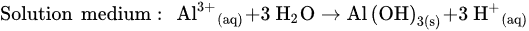
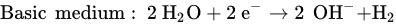
The reaction that happened in the cathode is a reduction in water based on different conditions, as follows.
In Equations, the Al3+ produced on the electrode react to form several species, including:
-
monomeric species such as Al(OH)2+, Al(OH)2+, Al2(OH)24+, and Al(OH)4−;
-
polymeric species such as Al6(OH)153+, Al7(OH)174+, Al8(OH)204+, Al13O4(OH)247+ and Al13(OH)345+;
-
amorphous species with very low solubility, such as Al(OH)3 and Al2O3 [14].
The formation of amorphous Al(OH)3, also called sweep flocs, needs surface areas which are useful for the adsorption of soluble pollutants and the trapping of the colloidal compounds [15]. The polymerization of flocs is as follows:
The flocs can be easily removed from an aqueous medium using the sedimentation method and H2 flotation. However, aluminium hydroxide usually acts as an adsorbent or traps the pollutants. It would be a great composition for the removal of the organic compound from the solution [16].
2. Type of Agro-Based Wastewater
2.1. Olive Oil Mill Wastewater
In general, olive oil mill wastewater (OOMW) consists of 80–83% water, 15–18% organic compounds, and 2% inorganic compounds [17]. OOMW has the characteristics of being dark in color, having an acidic pH (~5), and having highly toxic components including tannins, phenols and acid compounds making up about 37% of the total mass [18][19]. It has been reported that more than thirty types of phenolic compounds have been identified.
The electrocoagulation process can be used for OOMW because it decomposes the organic compound and is commonly used for OOMW treatment [20]. Electrocoagulation has stability for colloids, suspension and emulsion, which is affected by electric charge. When an electric charge is supplied into a suitable electrode, the compound will be neutralized and the rest of it will aggregate together into a larger and separable compound [21].
A study showed that electrocoagulation can remove COD by up to 78% after 1 h of electrolysis time using Fe as the electrode. The energy consumption reaches 55 kWh m−3. However, using Al as the electrode, the removal of COD has a lower value than that using Fe. The removal efficiency is only 55% for 1 h of electrolysis time. It also acquires higher energy consumption until 62 kWh m−3 [22]. The reduction of the energy consumption using Al as an electrode can be acheived by diluting the sample 10 times. The study reported that the energy consumption was decreased to 1 kWh/L with 57% COD removal [23]. The kinetic model of the electrocoagulation process using the one-factor method increases the performance of COD removal to 99% with conditions of 60 min electrolysis time, with the current density at 12.5 mA/cm2 and the addition of 400 mg/L NaCl [24]. The study conducted by Benekos [25] compared the performance of electrocoagulation on a laboratory scale and pilot scale. The results show on the laboratory scale that the COD and color removal (50% and 100%, respectively) is higher than that at the laboratory scale (42.5% and 85.3%, respectively).
The electrocoagulation process on OOMW is useful as a pre-treatment for biofuel production [21]. The methane production reaches 1902 kJ/LOOMW. The treatment of COD removal also produces biogas, as reported by a previous study. The result shows that the COD removal of OOMW using the electrocoagulation process produces biogas around 0.741 g/LCOD [26].
2.2. Sugar Industry Wastewater
The sugar industry categorized as one of the largest agro-based industries. It uses about 1500–2000 dm3 water and produces about 1000 dm3 wastewater per ton of processing [27]. The wastewater mainly results from the process of floor washing, condensation, leakage, and spillage of solutions from the pipeline and valve [28]. The sugar wastewater industries (SIWW) have a high concentration of organic materials due to the presence of sugar and organic material in the cane or beet. SIWW contains organic compounds including COD ranging between 2300 and 8000 mg/dm−3, BOD of about 1700–6600 mg/dm−3, and TSS of about 5000 mg/dm−3 [29]. In addition, SIWW also possibly contains pesticides, herbicides and pathogens from the contaminated material and processes which are produced [27].
The electrocoagulation process becomes one of the choices to be considered for SIWW treatment when the conventional method can not reduce the pollution [30]. The previous study reported that COD removal on SIWW treatment can reach 86.36% for 8 h of electrolysis time. The 12 V voltage is applied to the system without any pre-treatment. Using iron as an electrode increases the COD removal of SIWW, which reaches up to 84% for 2 h electrolysis time. The color reduction is about 86% with the current density of 178 A/m2. The lowest energy consumption utilized is 16.75 kWh/L. However, the performance of COD removal decreases to 62%. This result does not suffice for the final discharge of wastewater; therefore, further advanced methods could possibly be applied to bring the wastewater to meet the final discharge [31].
Different cases happened with simulated sugar industrial effluent using the research surface method to study the effects of various parameters using the research surface method (RSM). The maximum COD removal of about 83.94% is obtained with the energy consumption of 6.64 kWh kg−1. This system requires less energy.
2.3. Pulp and Paper Mill Wastewater
The pulp and paper industry produces highly polluted wastewater [32]. It forms black liquor as the main by-product, which contains about 50% lignin. The pulp and paper industry discards lignin to make a good quality of paper [33]. The release of lignin comes from the process of alkaline extraction at the bleaching stage. Lignin is a heterogeneous three-dimensional polymer that consists of oxy phenylpropanoid components. The contamination of phenolic compounds to the environment could damage the underground water and receiving water bodies [2].
The electrocoagulation process is one of the great alternatives to treat pulp and paper mill wastewater. Aluminium and iron are widely used as electrodes for the electrocoagulation process. A study showed that iron has greater efficiency for BOD and COD removal compared to aluminium. The performance of iron can reach up to the complete removal of COD and 90% removal of BOD within a 60 min reaction [34]. Other concerns for pulp and paper mill wastewater are lignin and phenol. By using iron as an electrode material, the electrochemical process can degrade lignin and phenol by up to 90% [29].
2.4. Palm Oil Mill Effluent
Palm oil mill effluent (POME)’s typical liquid waste results from the extraction of fresh fruit bunch (FFB) in the palm oil industry [35][36]. The characteristic of POME is a thick brownish liquid color due to the decomposition of lignocellulosic materials [37][38].
Some studies have found the performance of the electrochemical process for POME treatment. Using Al as the electrode shows little removal of COD and BOD. The percentage of COD and BOD removal is 30% and 38%, respectively [39]. However, the addition of NaNO3 could improve the performance of the electrochemical process using Al. The efficiency of COD removal increases by 64%. Using NaNO3 as the electrolyte support is also beneficial for subsequent biological treatment [40].
Another method uses iron for the electrochemical process. The performance of iron is quite great, with 89.2% removal of COD in 15 min of electrolysis time. For another good value, using iron as an electrode could remove the color by up to 90.4% [41]. In addition, steel wool can be used as an electrode. It could remove COD by up to 74% and BOD by up to 70% with the improvement of the electrode arrangement [42].
2.5. Coffee Industry Wastewater
Coffee is one of the world’s most well-known beverages, and it is the world’s second-most-traded commodity after petroleum [43]. The wastewater produced from the coffee processing industry ranges from 40 to 45 L for every kg of coffee [44]. The process requires large amounts of water for every step; therefore, the wastewater produced is high [45]. Coffee fruits themselves are rich in caffeine, sugars, phenolic compounds, fatty acids, lignin, cellulose, pectic substances and other macromolecules [46]. Those compounds are not suitable to be released into the environment due to their toxicity [47].
Coffee processing wastewater (CPWW) is characteristically black in color. It is high in persistent compounds known as melanoidins, which are toxic, recalcitrant and non-biodegradable [48]. Releasing the untreated CPWW to the water body can promote eutrophication, reduce Secchi depth and prevent sunlight penetration. The concentration of oxygen in the water body will be depleted [49]. The electrocoagulation process can be used for the decolorization of CPWW treatment [50]. The addition of electrolyte support such as sodium chloride affects the removal efficiency due to the generation of active chlorine during the electrolysis process interacting with substances such as melanoidins [51]. Other than meloinidins, the mineralization of proteins and lipids are adsorbed on the surface of the precipitated hydroxides [52].
Previous studies reported that the use of a combination of iron and stainless steel as electrodes achieves 87% COD removal and 97.1% color removal [53]. When using iron alone as an electrode material, it removes 97% of COD and 89% of color [54]. The electrocoagulation process can also be used for caffeine recovery that reaches 96% recovery performance [55]. However, there is still no report for polygalacturonase characterization and production in the coffee industry using the electrocoagulation process.
2.6. Vegetable Oil Refinery Wastewater
Vegetable oil refinery wastewater (VORW) has a high amount of COD, phosphorus, sulphate, oil and grease [56]. The sources of vegetable oil manufacture are soybeans, groundnut, rapeseed, sunflower, safflower, cotton, sesame, coconut, mustard, rice bran, watermelon, and neem, etc. [57]. Another characteristic of VORW is that it has a low ratio of BOD/COD; therefore, it is not useful for the biodegradation method. Based on some studies, electrochemical technology could overcome that limitation [58].
A previous study reported that using iron as the electrode could remove 93.3% of COD after 60 min electrolysis time [59]. However, when aluminium is used as an electrode, the COD removal increases up to 98.9%, with 100% color removal in 90 min [60]. Another study using the Box-Behnken design for optimization attained 70.8% COD removal. Aluminium was used as an electrode, and the electrolysis time was 60 min [61]. On the other hand, another study reported that using the Box-Behnken design for the optimization of the electrocoagulation process of sunflower oil refinery could achieve 95% COD removal with a shorter time (18 min) [62].
2.7. Nuts Processing Wastewater
The nut processing industry includes many various types, including pistachio, almond, and cashew nut, etc. Pistachio is a kind of nut rich in organic nutrients. It contains 5.6% water, 19.6% protein, 53.2% fat, 19% carbohydrate, and 2.6% ash [63]. The pistachio industry produces approximately 1 Mm3 of wastewater and 50,000 tons of solid waste per year. Pistachio processing wastewater (PPW) has high COD, phenolic compounds and turbidity, which can cause harm to the aquatic ecosystem and terrestrial environment if no treatment of the PPW is performed [64].
Several processes are required in the almond processing industry, including cracking and blanching [65]. In the blanching step, the resulting product contains high levels of organic compounds, suspended solids, turbidity, COD and color [66]. The effect of the blanching time on the moisture content is inversely proportional. This means that when the blanching time is longer, it results in a decreased moisture content. Meanwhile, the effect of the blanching time on the lipid content and color intensity is directly proportional. The longer blanching time will increase the lipid content and color intensity [67]. A previous study reported that the polyphenol content recovery in the almond industry using the blanching process acquired 0.53 g gallic acid equivalents (GAE)/kg [68], while the use of the microwave process recovered 0.42 g gallic acid equivalents (GAE)/kg [69]. Based on these studies, the polyphenol content recovery is higher using the blanching process. The use of blanching and microwave processes is recommended according to a previous study.
The kinetic study showed that the sonicated almond acquires higher total phenolic compounds than non-sonicated almonds after 20 min extraction at room temperature (25 °C) [70]. Table 1 shows the performance of the electrocoagulation process on different types of nut processing wastewater. The electrocoagulation process has been shown to be a suitable method for the reduction of pollutants from cashew nut processing wastewater, as was reported in a previous work where COD reduced it by up to 80% [71]. According to a previous study, even though the COD removal for PPW is categorized as low, the phenolic compound removal shows greater efficiencies. In addition, using graphite as the electrode material on PPW could achieve the complete removal of phenolic compounds and the 99.79% removal of COD [72].
Table 1. Performance of the electrochemical process on nut processing wastewater.
| Type of Nuts | Electrode Material | Electrode Type | COD removal | Phenolic removal | References |
|---|---|---|---|---|---|
| Pistachio processing industry wastewater | Al | Unipolar | 60.1% | 77.3% | [73] |
| Pistachio processing industry wastewater | Graphite | Unipolar | 99.79% | 100% | [74] |
| Pistachio processing industry wastewater | Al | Unipolar | 57.4% | - | [65] |
| Pistachio processing industry wastewater | Al and stainless steel | Unipolar | 60% | 95% | [74] |
| Cashew nut processing industry wastewater | Fe, BDD (Boron Doped-Diamond) and stainless steel | Multipolar | 80% | - | [75] |
References
- Markou, V.; Kontogianni, M.; Frontistis, Z.; Tekerlekopoulou, A.G.; Katsaounis, A.; Vayenas, D. Electrochemical treatment of biologically pre-treated dairy wastewater using dimensionally stable anodes. J. Environ. Manag. 2017, 202, 217–224.
- Sahu, O.P.; Chaudhari, P.K. Electro-chemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129.
- Klidi, N.; Clematis, D.; Delucchi, M.; Gadria, A.; Ammara, A.; Panizza, M. Applicability of electrochemical methods to paper mill wastewater for reuse:Anodic oxidation with BDD and TiRuSnO2 anodes. J. Electroanal. Chem. 2018, 815, 16–23.
- Ellouze, S.; Panizza, M.; Barbucci, A.; Cerisola, G.; Mhiria, T.; Elaouda, S.C. Ferulic acid treatment by electrochemical oxidation using a BDD anode. J. Taiwan Inst. Chem. Eng. 2016, 59, 132–137.
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126–198.
- Nowacka, A.; Makula, M.M.; Macherzynski, B. Comparison of effectiveness of coagulation with aluminum sulfate and pre-hydrolyzed aluminum coagulants. Desalin. Water Treat. 2014, 52, 3843–3851.
- Ghernaout, D.; Ghernaout, B.; Kellil, A. Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. Desalin. Water Treat. 2009, 2, 203–222.
- Guminska, J.; Kłos, M. Analysis of post-coagulation properties of flocs in terms of coagulant choice. Environ. Prot. Eng. 2012, 38, 103–113. Available online: https://www.infona.pl/resource/bwmeta1.element.baztech-article-BPW8-0022-0035 (accessed on 14 November 2021).
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2017, 33, 263–292.
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21.
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168.
- Dura, A.; Breslin, C. Electrocoagulation for the Effective Removal of Pollutants. ECS Meet. Abstr. 2012, 1417. Available online: https://mural.maynoothuniversity.ie/6744/1/adelaide-dura.pdf (accessed on 15 November 2021).
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Hecini, M.; Hamitouche, H. Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem. Eng. Processing Process Intensif. 2010, 49, 1176–1182.
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663.
- Yassine, W.; Akazdam, S.; Zyade, S.; Gourich, B. Treatment of olive mill wastewater using electrocoagulation process. J. Appl. Surf. Interfaces 2019, 4, 24–30. Available online: https://revues.imist.ma/index.php/jasi/article/view/14006 (accessed on 10 November 2021).
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Al-Qaim, F.F.; Purba, L.D.A.; Riyadi, F.A. Application of Box-Behnken design to mineralization and color removal of palm oil mill effluent by electrocoagulation process. Environ. Sci. Pollut. Res. 2021, 1–13.
- Chiang, L.C.; Chang, J.E.; Tseng, S.C. Electrochemical oxidation pretreatment of refractory organic pollutants. Water Sci. Technol. 1997, 36, 123–130.
- Niaounakis, M.; Halvadakis, C.P. Olive-Mill Waste Management, 2nd ed.; Typothito: Athens, Greece, 2006; Available online: https://www.sciencedirect.com/bookseries/waste-management-series/vol/5/suppl/C (accessed on 11 November 2021).
- Dellagreca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular-weight components of olive oil mill waste-waters. Phytochem. Anal. 2004, 15, 184–188.
- Sounni, F.; Aissam, H.; Ghomari, O.; Merzouki, M.; Benlemlih, M. Electrocoagulation of olive mill wastewaters to enhance biogas production. Biotechnol. Lett. 2018, 40, 297–301.
- Ghahrchi, M.; Rezaee, A.; Adibzadeh, A. Study of kinetic models of olive oil mill wastewater treatment using electrocoagulation process. Desalin. Water Treat. 2021, 211, 123–130.
- Razali, N.A.M.; Salleh, W.N.W.; Rosman, N.; Ismail, N.H.; Ahmad, S.Z.N.; Aziz, F.; Jye, L.W.; Ismail, A.F. Palm oil mill effluent treatment using tungsten trioxide: Adsorption and photocatalytic degradation. Mater. Today Proc. 2021, 42, 22–27.
- Longhi, P.; Vodopivec, B.; Fiori, G. Electrochemical treatment of olive oil mill wastewater. Ann. Chim. 2001, 91, 169–174.
- Tezcan-Un, U.; Ugur, S.; Koparal, A.S.; Bakir-Ogutveren, U. Electrocoagulation of olive mill wastewaters. Sep. Purif. Technol. 2006, 52, 136–141.
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water 2020, 13, 11.
- Benekos, A.K.; Zampeta, C.; Argyriou, R.; Economou, C.N.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Tekerlekopoulou, A.G.; Vayenas, D.G. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process Saf. Environ. Prot. 2019, 131, 38–47.
- Ntaikou, I.; Antonopoulou, G.; Vayenasa, D.; Lyberatosa, G. Assessment of electrocoagulation as a pretreatment method of olive mill wastewater towards alternative processes for biofuels production. Renew. Energy 2020, 154, 1252–1262.
- Sahu, O.P.; Gupta, V.; Chaudhari, P.K.; Srivastava, V.V. Electrochemical treatment of actual sugar industry wastewater using aluminum electrodes. Int. J. Environ. Sci. Technol. 2015, 12, 3519–3530.
- Asaithambhi, P.; Matheswaran, M. Electrochemical treatment of simulated sugar industrial effluent: Optimization and modeling using a response surface methodology. Arab. J. Chem. 2016, 9, 981–987.
- Kolhe, A.S.; Sarode, A.G.; Ingale, S.R. Study of effluent from the sugar cane industry. Sodh Samiksha Aur Mulyankan 2009, 2, 303–306. Available online: https://www.researchgate.net/publication/351249954_Quality_and_Treatment_of_Sugar_Industry_Effluent_-A_Study (accessed on 12 November 2021).
- Guven, G.; Perendeci, A.; Tanylac, A. Electrochemical treatment of simulated beet sugar factory wastewater. Chem. Eng. J. 2009, 151, 149–159.
- Brillas, E.; Sauleda, R.; Casado, J. Degradation of4-chlorophenol by anodic oxida- tion, electro-Fenton, photoelectro-Fenton and peroxi-coagulation processes. J. Electrochem. Soc. 1998, 145, 759–765.
- Chindaphan, K.; Thaveesangsakulthai, I.; Naranaruemol, S.; Nhujak, T.; Panchompoo, J.; Chailapakol, O.; Kulsing, C. Miniaturized electrocoagulation approach for removal of polymeric pigments and selective analysis of non- and mono-hydroxylated phenolic acids in wine with HPLC-UV. RSC Adv. 2021, 11, 5885–5893.
- Ksibi, M.; Amor, S.B.; Cherif, S.; Elaloui, S. Photodegradation of lignin from black liquor using a UV/TiO2 system. J. Photochem. Photobiol. A 2003, 154, 211–218.
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents: A review. Adv. Environ. Res. 2001, 5, 175–196.
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Rezania, S.; Khademi, T.; Kumar, A. Palm oil mill effluent as an environmental pollutant. Palm Oil 2018, 13, 13–28. Available online: https://www.intechopen.com/chapters/60482 (accessed on 13 November 2021).
- Zazouli, M.A.; Ahmadi, M. Pretreatment of paper recycling plant wastewater by electrocoagulation using aluminum and iron electrodes. J. Mater. Environ. Sci. 2017, 8, 2140–2146.
- Ugurlu, M.; Gurses, A.; Dogar, C.; Yalcin, M. The removal of lignin and phenol from paper mill effluents by electrocoagulation. J. Environ. Manag. 2007, 87, 420–428.
- Chin, M.J.; Eong, P.P.; Ti, T.B.; Seng, C.E.; Ling, C.K. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726.
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Shahbazian, R.Y.; Rezania, S.; Khademi, T.; Azimi, M. Evaluation of Lemna minor and Chlamydomonas to treat palm oil mill effluent and fertilizer production. J. Water Process Eng. 2017, 17, 229–236.
- Agustin, M.B.; Sengpracha, W.P.; Phutdhawong, W. Electrocoagulation of Palm Oil Mill Effluent. Int. J. Environ. Res. Public Health 2008, 5, 177–180.
- Phalakornkule, C.; Mangmeemak, J.; Intrachod, K.; Nuntakumjorn, B. Pretreatment of palm oil mill effluent by electrocoagulation and coagulation. ScienceAsia 2010, 36, 142–149.
- Bouknana, D.; Hammoutia, B.; Salghid, R.; Jodehe, S.; Zarrouka, A.; Warade, I.; Aouniti, A.; Sbaa, M. Physicochemical Characterization of Olive Oil Mill Wastewaters in the eastern region of Morocco. J. Mater. Environ. Sci. 2014, 5, 1039–1058.
- Phan, H.Q.H.; Hoan, N.X.; Huy, N.N.; Duc, N.D.D.; Anh, N.T.N.; Que, N.T.; Thuy, N.T. Pre-treatment potential of electrocoagulation process using aluminum and titanium electrodes for instant coffee processing wastewater. J. Environ. Sustain. 2019, 3, 170–185.
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative techniques for caffeine removal from wastewater: An overview of opportunities and challenges. J. Water Proc. Eng. 2020, 35, 101231.
- Gomez, I.D.; Garcia, M.A.G. Integration of environmental and economic performance of Electro-Coagulation-Anodic Oxidation sequential process for the treatment of soluble coffee industrial effluent. Sci. Total Environ. 2021, 764, 142818.
- Sontaya, K.; Pitiyont, B.; Punsuvon, P. Decolorization and COD Removal of Palm Oil Mill Wastewater by Electrocoagulation. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2013, 7, 606–609.
- Nasrullah, M.; Singh, L.; Krishnan, S.; Sakinah, M.; Zularism, A.W. Electrode design for electrochemical cell to treat palm oil mill effluent by electrocoagulation process. Environ. Technol. Innov. 2018, 9, 323–341.
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58.
- Rodríguez, P.S.; Pérez, S.R.M.; Fern¢ndez, B.M. Studies of anaerobic biodegradability of the wastewater of the humid benefit the coffee. J. Interciencia 2000, 25, 386–390. Available online: https://www.researchgate.net/publication/297388993_Study_of_the_anaerobic_biodegradability_of_the_wastewaters_of_the_humid_benefit_of_the_coffee (accessed on 11 November 2021).
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374.
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429.
- Gengec, E.; Kobya, M.; Demirbas, E.; Akyol, A.; Oktor, K. Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination 2012, 286, 200–209.
- Khansorthong, S.; Hunsom, M. Remediation of wastewater from pulp and paper mill industry by the electrochemical technique. J. Chem. Eng. 2009, 151, 228–234.
- Sahana, M.; Srikantha, H.; Mahesh, H.; Swamy, M.H. Coffee processing industrial wastewater treatment using batch electrochemical coagulation with stainless steel and Fe electrodes and their combinations, and recovery and reuse of sludge. Water Sci. Technol. 2018, 78, 279–289.
- Asha, G.; Kumar, B.M. Comparison of Aluminum and Iron Electrodes for COD Reduction from Coffee Processing Wastewater by Electrocoagulation Process. J. Sci. Res. Rep. 2016, 9, 22815.
- Waldron, K.W.; Moates, G.K.; Faulds, C.B. Prebiotic Potential and Antimicrobial Effect from a By-Product of the Almond Processing Industry. In Total Food; RSC Publishing: Cambridge, UK, 2009; pp. 86–89.
- Pandey, R.A.; Sanyal, P.B.; Chattopadhyay, N.; Kaul, S.N. Treatment and reuse of wastes of a vegetable oil refinery. Resour. Conserv. Recycl. 2003, 37, 101–117.
- Bucas, G.; Saliot, A. Sea transport of animal and vegetable oils and its environmental consequences. Mar. Pollut. Bull. 2002, 44, 1388–1396.
- Sridhar, S.; Kale, A.; Khan, A.A. Reverse osmosis of edible vegetable oil industry effluent. J. Membr. Sci. 2002, 205, 83–90.
- Tezcan-Un, U. Treatment of vegetable oil refinery wastewater by electrocoagulation. Fresenius Environ. Bull. 2007, 16, 1056–1060.
- Tezcan-Un, U.; Koparal, A.S.; Ogutveren, U.B. Electrocoagulation of vegetable oil refinery wastewater using aluminum electrodes. J. Environ. Manag. 2009, 90, 428–433.
- Preeti, V.; Ramesh, S.T.; Gandhimathi, R.; Nidhessh, P.V. Optimization of batch electrocoagulation process using Box-Behnken experimental design for the treatment of crude vegetable oil refinery wastewater. J. Dispers. Sci. Technol. 2019, 41, 592–599.
- Sharma, S.; Aygun, A.; Simsek, H. Electrochemical treatment of sunflower oil refinery wastewater and optimization of the parameters using response surface methodology. Chemosphere 2020, 249, 126511.
- Bayar, S.; Boncukcouglu, R.; Yilmaz, A.E.; Fil, B.A. Pre-Treatment of Pistachio Processing Industry Wastewaters (PPIW) by Electrocoagulation using Al Plate Electrode. J. Sep. Sci. Technol. 2014, 49, 1008–1018.
- Celik, I.; Demirer, G.N. Biogas production from pistachio (pistacia vera L.) processing waste. Biocatal. Agric. Biotechnol. 2015, 4, 767–772.
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzman, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT 2018, 89, 299–306.
- Lipan, L.; Moriana, A.; Lluch, D.B.L.; Lamadrid, M.C.; Sendra, E.; Hernandez, F.; Araujo, L.V.; Corell, M.; Barrachina, A.A.C. Nutrition Quality Parameters of Almonds as Affected by Deficit Irrigation Strategies. Molecules 2019, 24, 2646.
- Bodoira, R.; MAestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–948.
- Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Appl. Sci. 2020, 10, 3628.
- Valero, D.; Ortiz, J.M.; García, V.; Expósito, E.; Montiel, V.; Grupo, A.A. Electrocoagulation of wastewater from the almond industry. Chemosphere 2011, 84, 1290–1295.
- Costa, P.R.F.D.; Costa, E.C.T.D.A.; Castro, S.S.L.; Fajardo, A.S.; Huitle, C.A.M. A sequential process to treat a cashew-nut effluent: Electrocoagulation plus electrochemical oxidation. J. Electroanal. Chem. 2019, 834, 79–85.
- Jaafarzadeh, N.; Omidinasab, M.; Ghanbari, F. Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Saf. Environ. Prot. 2016, 102, 462–472.
- Fil, B.A.; Boncukcouglu, R.; Yilmaz, A.E.; Bayar, S. Electro-Oxidation of Pistachio Processing Industry Wastewater Using Graphite Anode. Soil Air Water 2014, 42, 1232–1238.
- Guclu, D. Optimization of electrocoagulation of pistachio processing wastewaters using the response surface methodology. Desalin. Water Treat. 2015, 54, 3338–3347.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
28 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




