
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hidesaku Asakura | + 3551 word(s) | 3551 | 2022-03-21 04:34:25 | | | |
| 2 | Vivi Li | Meta information modification | 3551 | 2022-03-21 10:43:48 | | |
Video Upload Options
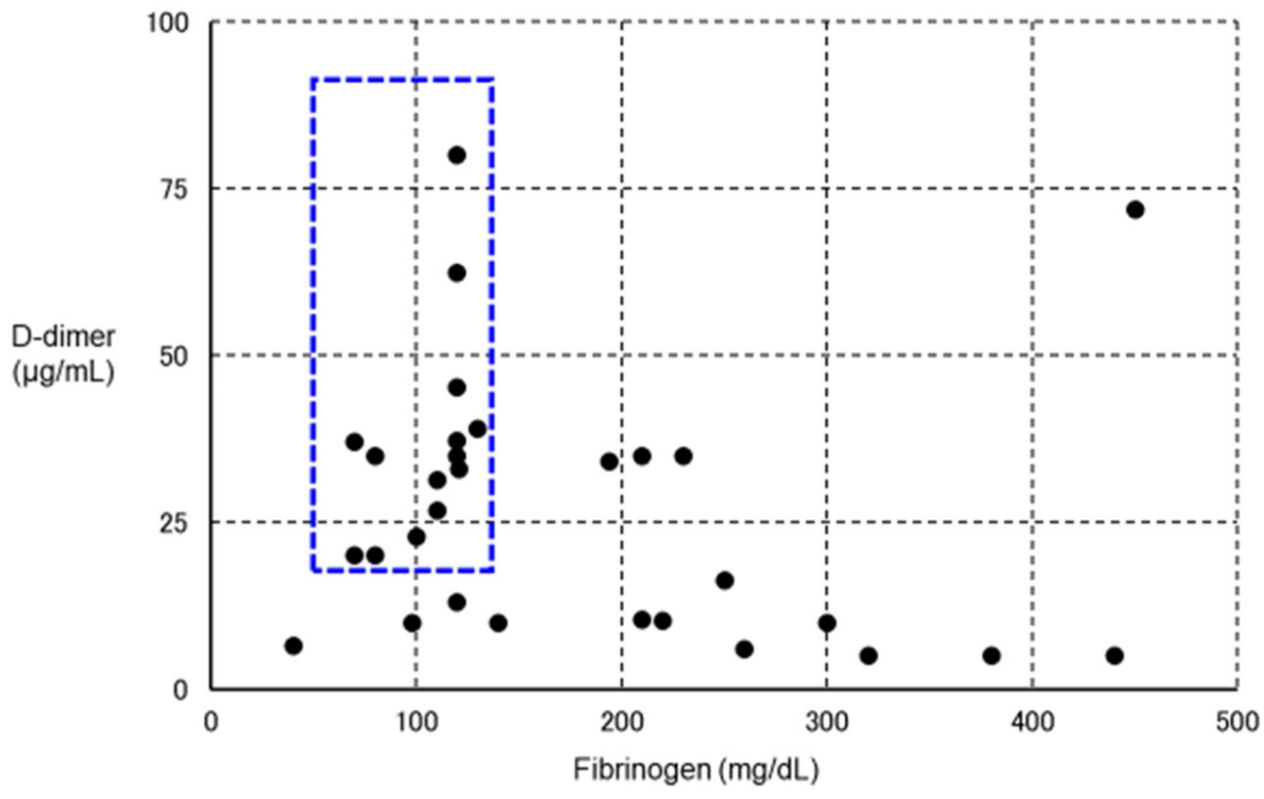
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is frequently complicated by thrombosis. In some cases of severe COVID-19, fibrinolysis may be markedly enhanced within a few days, resulting in fatal bleeding. In the treatment of COVID-19, attention should be paid to both coagulation activation and fibrinolytic activation. Various thromboses are known to occur after vaccination with SARS-CoV-2 vaccines. Vaccine-induced immune thrombotic thrombocytopenia (VITT) can occur after adenovirus-vectored vaccination, and is characterized by the detection of anti-platelet factor 4 antibodies by enzyme-linked immunosorbent assay and thrombosis in unusual locations such as cerebral venous sinuses and visceral veins. Treatment comprises high-dose immunoglobulin, argatroban, and fondaparinux. Some VITT cases show marked decreases in fibrinogen and platelets and marked increases in D-dimer, suggesting the presence of enhanced-fibrinolytic-type disseminated intravascular coagulation with a high risk of bleeding. In the treatment of VITT, evaluation of both coagulation activation and fibrinolytic activation is important, adjusting treatments accordingly to improve outcomes.
1. Introduction

2. SARS-CoV-2 Vaccination-Associated Coagulopathy
2.1. Safety of Vaccination in Persons with Coagulation Abnormalities
2.1.1. Preventing Bleeding Due to Vaccination in Patients with Coagulation Abnormalities
2.1.2. Vaccination and Thrombotic/Hemorrhagic Disease Exacerbations and Relapses
2.2. Novel Clotting Abnormalities after Vaccination
2.2.1. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
2.2.2. Thrombotic/Bleeding Disorders Other Than VITT
| Disease Name | Abbreviation | Important Clinical and Laboratory Findings |
|---|---|---|
| Heparin-induced thrombocytopenia | HIT | History of exposure to heparin, 4T’s score |
| Thrombotic microangiopathy | TMA | Appearance of schizocytes (peripheral blood smear), marked decrease in haptoglobin |
| Thrombotic thrombocytopenic purpura | TTP | A type of TMA with markedly reduced ADAMTS13 activity with ADAMTS13 inhibitor |
| Immune thrombocytopenia | ITP | Diagnosis of exclusion. Increased megakaryocytes in bone marrow and positive antiplatelet antibodies assist in diagnosis |
| Antiphospholipid antibody syndrome | APS | Positive for at least one of the following antibodies: lupus anticoagulant; anticardiolipin antibody; and anti-β2 GPI antibody |
| Paroxysmal nocturnal hemoglobinuria | PNH | Hemolysis (normocytic anemia, elevated reticulocyte, elevated indirect bilirubin, elevated LDH, decreased haptoglobin), presence of PNH type-cells (CD55/59-negative) |
| Disseminated intravascular coagulation | DIC | PT, APTT, fibrinogen, FDP, D-dimer, AT, TAT, PIC, plasminogen, α2PI |
- (1)
-
Hemorrhagic disease
- (2)
-
Thrombotic disease
- (3)
-
Thrombophilia with low platelet count
3.3. Pathophysiology of VITT

3.4. Diagnosis of VITT
| Clinical Findings |
|---|
| (1) Onset 4–28 days after vaccination (counting the day of vaccination as day 0) |
| (2) Symptoms suggestive of stroke (unilateral facial palsy, unilateral motor palsy, language disorder, joint parallax, hemispheric neglect, etc.) |
| (3) Symptoms suggestive of cerebral venous sinus thrombosis (persistent headache, visual disturbance, seizure, nausea and vomiting, psychiatric symptoms, etc.) |
| (4) Symptoms suggestive of visceral vein thrombosis (persistent abdominal pain, nausea and vomiting, etc.) |
| (5) Symptoms suggestive of deep vein thrombosis or pulmonary thromboembolism (pain and swelling in lower limbs, chest and back pain, shortness of breath, etc.) |
| (6) Hemorrhagic tendencies such as hemorrhagic infarction, petechial hemorrhage, and mottled hemorrhage can also occur. |
3.5. Treatment of VITT (Attention to Fibrinolytic Pathophysiology)
| Disease | Suppressed-Fibrinolytic-Type DIC | Enhanced-Fibrinolytic-Type DIC | VITT | |
|---|---|---|---|---|
| Underlying disease/cause | Severe sepsis | APL, aortic aneurysm, prostate cancer, etc. | Adenovirus vector type vaccination |
|
| Pathophysiology | Activation of coagulation and mild fibrinolysis activation | Activation of coagulation and enhanced fibrinolysis | Antibodies against PF4 are mediated platelet and coagulation activation |
|
| Main symptom | Organ damage | Bleeding | Headache, abdominal pain, etc. | |
| Examination findings | Platelet count | Decreased | Decreased | Decreased |
| PT | Prolonged | Normal to prolonged | Normal to prolonged * | |
| APTT | Prolonged | Slightly shortened to prolonged | Normal to prolonged * | |
| Fibrinogen | Normal to elevated | Decreased | Significantly reduced to normal | |
| D-dimer | Increased | Increased | Increased | |
| FDP | Increased | Markedly increased | Increased—markedly increased * | |
| TAT | Increased | Increased | Increased * | |
| PIC | Slightly increased | Markedly increased | Increased—markedly increased * | |
| Medical treatment | Anticoagulant therapy | Anticoagulant therapy ± antifibrinolytic therapy | Anticoagulant therapy other than heparin, high-dose immunoglobulin therapy, etc. | |
References
- Geerts, W.; Selby, R. Prevention of Venous Thromboembolism in the ICU. Chest 2003, 124, 357S–363S.
- Horiuchi, H.; Morishita, E.; Urano, T.; Yokoyama, K. The Questionnaire-survey Joint Team on The COVID-19-related thrombosis COVID-19-Related Thrombosis in Japan: Final Report of a Questionnaire-Based Survey in 2020. J. Atheroscler. Thromb. 2021, 28, 406–416.
- Kerbikov, O.; Orekhov, P.; Borskaya, E.; Nosenko, N. High incidence of venous thrombosis in patients with moderate-to-severe COVID-19. Int. J. Hematol. 2021, 113, 344–347.
- Hunter, P.R. Thrombosis after COVID-19 vaccination. BMJ 2021, 373, n958.
- Perrin, G.; Le Beller, C.; Darnige, L.; Khider, L.; Smadja, D.M.; Louet, A.L.-L.; Planquette, B.; Lebeaux, D.; Sanchez, O.; Sabatier, B.; et al. Intramuscular Vaccination in Adults with Therapeutic Anticoagulation in the Era of COVID-19 Vaccines Outbreak: A Practical Review. TH Open 2021, 5, e166–e170.
- Ogawa, H.; Asakura, H. Consideration of Tranexamic Acid Administration to COVID-19 Patients. Physiol. Rev. 2020, 100, 1595–1596.
- Yamada, S.; Asakura, H. Therapeutic Strategies for Disseminated Intravascular Coagulation Associated with Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 2396.
- Jiang, D.; Portuguese, A.J.; Weatherford, A.; Garcia, D.; Gernsheimer, T. Platelet trends after Covid-19 vaccination in patients with chronic or persistent immune thrombocytopenia. Am. J. Hematol. 2021, 96, E472–E474.
- Visser, C.; Swinkels, M.; van Werkhoven, E.D.; Croles, F.N.N.; Noordzij-Nooteboom, H.S.; Eefting, M.; Last-Koopmans, S.M.; Idink, C.; Westerweel, P.E.; Santbergen, B.; et al. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022, 6, 1637–1644.
- Lee, E.-J.; Beltrami-Moreira, M.; Al-Samkari, H.; Cuker, A.; DiRaimo, J.; Gernsheimer, T.; Kruse, A.; Kessler, C.M.; Kruse, C.; Leavitt, A.D.; et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood 2022, 139, 1564–1574.
- Crickx, E.; Moulis, G.; Ebbo, M.; Terriou, L.; Briantais, A.; Languille, L.; Limal, N.; Guillet, S.; Michel, M.; Mahevas, M.; et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br. J. Haematol. 2021, 195, 703–705.
- Kuter, D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021, 195, 365–370.
- Immune thrombocytopenia associated with COVID-19 mRNA vaccine tozinameran—a clinical case and global pharmacovigilance data. Swiss Med Wkly. 2021, 151, w30084.
- Gerber, G.F.; Yuan, X.; Yu, J.; Cher, B.A.Y.; Braunstein, E.M.; Chaturvedi, S.; Brodsky, R.A. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood 2021, 137, 3670–3673.
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 28 February 2022).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101.
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130.
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211.
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.G.; Weißenborn, K.; Höglinger, G.U.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353.
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456.
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965.
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine–induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570.
- Waraich, A.; Williams, G. Haematuria, a widespread petechial rash, and headaches following the Oxford AstraZeneca ChAdOx1 nCoV-19 Vaccination. BMJ Case Rep. 2021, 14, e245440.
- Paulsen, F.-O.; Schaefers, C.; Langer, F.; Frenzel, C.; Wenzel, U.; Hengel, F.E.; Bokemeyer, C.; Seidel, C. Immune Thrombocytopenic Purpura after vaccination with COVID-19 Vaccine (ChAdOx1 nCov-19). Blood 2021, 138, 996–999.
- Choi, P.Y.-I.; Hsu, D.; Tran, H.A.; Tan, C.W.; Enjeti, A.; Chen, V.M.Y.; Chong, B.H.; Curnow, J.; Pepperell, D.; Bird, R. Immune thrombocytopenia following vaccination during the COVID-19 pandemic. Haematologica 2021.
- Tarawneh, O.; Tarawneh, H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am. J. Hematol. 2021, 96, E133–E134.
- Nakamura, T.; Morodomi, Y.; Kanaji, S.; Okamura, T.; Nagafuji, K.; Kanaji, T. Detection of anti-GPIbα autoantibodies in a case of immune thrombocytopenia following COVID-19 vaccination. Thromb. Res. 2021, 209, 80–83.
- Okada, Y.; Sakai, R.; Sato-Fitoussi, M.; Nodera, M.; Yoshinaga, S.; Shibata, A.; Kurasawa, T.; Kondo, T.; Amano, K. Potential Triggers for Thrombocytopenia and/or Hemorrhage by the BNT162b2 Vaccine, Pfizer-BioNTech. Front. Med. 2021, 8, 751598.
- Hidaka, D.; Ogasawara, R.; Sugimura, S.; Fujii, F.; Kojima, K.; Nagai, J.; Ebata, K.; Okada, K.; Kobayashi, N.; Ogasawara, M.; et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2021, 115, 424–427.
- Radwi, M.; Farsi, S. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1515–1518.
- Shimoyama, S.; Kanisawa, Y.; Ono, K.; Souri, M.; Ichinose, A. First and fatal case of autoimmune acquired factor XIII /13 deficiency after COVID -19/ SARS-CoV -2 vaccination. Am. J. Hematol. 2021, 97, 243–245.
- Goereci, Y.; Kleineberg, N.N.; Madlener, M.; Neuschmelting, H.; Fink, G.R.; Warnke, C.; Stetefeld, H. Successful treatment of thromboses of major arteries after ChAdOx1 nCov-19 vaccination. Neurol. Res. Pract. 2021, 3, 1–4.
- Andraska, E.A.; Kulkarni, R.; Chaudhary, M.; Sachdev, U. Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 14–17.
- Carli, G.; Nichele, I.; Ruggeri, M.; Barra, S.; Tosetto, A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021, 16, 803–804.
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285.
- Varona, J.F.; García-Isidro, M.; Moeinvaziri, M.; Ramos-López, M.; Fernández-Domínguez, M. Pri-mary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur J Intern. Med. 2021, 91, 90–92.
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1771–1775.
- Shimazawa, R.; Ikeda, M. Potential adverse events in Japanese women who received tozinameran (BNT162b2, Pfizer-BioNTech). J. Pharm. Policy Pract. 2021, 14, 46.
- Kirpalani, A.; Garabon, J.; Amos, K.; Patel, S.; Sharma, A.P.; Ganesan, S.L.; Barton, M.; Cacciotti, C.; Leppington, S.; Bakovic, L.; et al. Thrombotic thrombocytopenic purpura temporally associated with BNT162b2 vaccination in an adolescent successfully treated with caplacizumab. Br. J. Haematol. 2021, 196, e11–e14.
- de Bruijn, S.; Maes, M.; De Waele, L.; Vanhoorelbeke, K.; Gadisseur, A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 2014–2018.
- Maayan, H.; Kirgner, I.; Gutwein, O.; Herzog-Tzarfati, K.; Rahimi-Levene, N.; Koren-Michowitz, M.; Blickstein, D. Acquired thrombotic thrombocytopenic purpura: A rare disease associated with BNT162b2 vaccine. J. Thromb. Haemost. 2021, 19, 2314–2317.
- Aladdin, Y.; Algahtani, H.; Shirah, B. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death following the ChAdOx1 nCoV-19 Vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105938.
- Al-Ali, D.; Elshafeey, A.; Mushannen, M.; Kawas, H.; Shafiq, A.; Mhaimeed, N.; Mhaimeed, O.; Mhaimeed, N.; Zeghlache, R.; Salameh, M.; et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell. Mol. Med. 2021, 26, 636–653.
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607.
- Vayne, C.; Rollin, J.; Gruel, Y.; Pouplard, C.; Galinat, H.; Huet, O.; Mémier, V.; Geeraerts, T.; Marlu, R.; Pernod, G.; et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 376–378.
- Tiede, A.; Althaus, K.; Sachs, U.J.; Cooper, N.; Czwalinna, A.; Müller, J.; Pötzsch, B. PF4-Dependent Immunoassays in Patients with Vaccine-Induced Immune Thrombotic Thrombocytopenia: Results of an Interlaboratory Comparison. Thromb. Haemost. 2021, 121, 1622–1627.
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256.
- Terpos, E.; Politou, M.; Ntanasis-Stathopoulos, I.; Karalis, V.; Merkouri, E.; Fotiou, D.; Gavriatopoulou, M.; Malandrakis, P.; Kastritis, E.; Trougakos, I.; et al. High Prevalence of Anti-PF4 Antibodies Following ChAdOx1 nCov-19 (AZD1222) Vaccination Even in the Absence of Thrombotic Events. Vaccines 2021, 9, 712.
- Hursting, M.J.; Pai, P.J.; McCracken, J.E.; Hwang, F.; Suvarna, S.; Lokhnygina, Y.; Bandarenko, N.; Arepally, G.M. Platelet Factor 4/Heparin Antibodies in Blood Bank Donors. Am. J. Clin. Pathol. 2010, 134, 774–780.
- Arepally, G.M.; Hursting, M.J. Platelet factor 4/heparin antibody (IgG/M/A) in healthy subjects: A literature analysis of commercial immunoassay results. J. Thromb. Thrombolysis 2008, 26, 55–61.
- Barefah, A.S.; Radhwi, O.O.; Alamri, S.S.; Alahwal, H.M.; Denetiu, I.; Almohammadi, A.T.; Bahashwan, S.M.; Qari, M.H.; Algaissi, A.; Alamer, E.; et al. Low clinical utility of testing for anti-platelet factor 4 in asymptomatic individuals after ChAdOx1 nCoV-19 vaccine. Int. J. Lab. Hematol. 2021.
- Yamaguchi, Y.; Kimihira, L.; Nagasawa, H.; Seo, K.; Wada, M. Cerebral Venous Sinus Thrombosis After BNT162b2 mRNA COVID-19 Vaccination. Cureus 2021, 13, e18775.
- Fan, B.E.; Shen, J.Y.; Lim, X.R.; Tu, T.M.; Chang, C.C.R.; Khin, H.S.W.; Koh, J.S.; Rao, J.P.; Lau, S.L.; Tan, G.B.; et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: A black swan event. Am. J. Hematol. 2021, 96, E357–E361.
- Dias, L.; Soares-Dos-Reis, R.; Meira, J.; Ferrão, D.; Soares, P.R.; Pastor, A.; Gama, G.; Fonseca, L.; Fagundes, V.; Carvalho, M. Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105906.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593.
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571.
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175.
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT)—Update on diagnosis and management considering different resources: Response to Comment from Yamada et al. J. Thromb. Haemost. 2022, 20, 542–543.
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources. J. Thromb. Haemost. 2022, 20, 149–156.
- Nazy, I.; Sachs, U.J.; Arnold, D.M.; McKenzie, S.E.; Choi, P.; Althaus, K.; Ahlen, M.T.; Sharma, R.; Grace, R.F.; Bakchoul, T. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J. Thromb. Haemost. 2021, 19, 1585–1588.
- Bussel, J.B.; Connors, J.M.; Cines, D.B.; Cines, D.B.; Dunbar, C.E.; Michaelis, L.C.; Kreuziger, L.B.; Lee, A.Y.Y.; Pabinger-Fasching, I. Throm-Bosis with Thrombocytopenia Syndrome (Also Termed Vaccine Induced Thrombotic Thrombocytopenia). Version 1.7. Available online: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenialast (accessed on 17 December 2021).
- Salih, F.; Schönborn, L.; Kohler, S.; Franke, C.; Möckel, M.; Dörner, T.; Bauknecht, H.C.; Pille, C.; Graw, J.A.; Alonso, A.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021, 385, 2103–2105.
- Khuhapinant, A.; Rungjirajittranon, T.; Suwanawiboon, B.; Chinthammitr, Y.; Ruchutrakool, T. Successful venous thromboprophylaxis in a patient with vaccine-induced immune thrombotic thrombocytopenia (VITT): A case report of the first reported case in Thailand. Thromb. J. 2021, 19, 1–5.
- Kennedy, V.E.; Wong, C.C.; Hong, J.M.; Peng, T.A.; Brondfield, S.; Reilly, L.M.; Cornett, P.; Leavitt, A.D. VITT following Ad26.COV2.S vaccination presenting without radiographically demonstrable thrombosis. Blood Adv. 2021, 5, 4662–4665.
- Johansen, S.; Lægreid, I.J.; Ernstsen, S.L.; Azrakhsh, N.A.; Kittang, A.O.; Lindås, R.; Gjertsen, B.T.; Vetti, N.; Mørtberg, T.V.; Sørvoll, I.H.; et al. Thrombosis and thrombocytopenia after HPV vaccination. J. Thromb. Haemost. 2021, 20, 700–704.
- Hwang, J.; Park, S.H.; Lee, S.W.; Lee, S.B.; Lee, M.H.; Jeong, G.H.; Kim, M.S.; Kim, J.Y.; Koyanagi, A.; Jacob, L.; et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: The FAPIC score. Eur. Heart J. 2021, 42, 4053–4063.
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2021, 9, e73–e80.
- Arepally, G.M.; Ortel, T.L. Vaccine-induced immune thrombotic thrombocytopenia: What we know and do not know. Blood 2021, 138, 293–298.
- Thaler, J.; Ay, C.; Gleixner, K.V.; Hauswirth, A.W.; Cacioppo, F.; Grafeneder, J.; Quehenberger, P.; Pabinger, I.; Knöbl, P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J. Thromb. Haemost. 2021, 19, 1819–1822.
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensiv. Care 2014, 2, 20.
- Wilting, F.N.; Kotsopoulos, A.M.; Platteel, A.C.; van Oers, J.A. Intracerebral Hemorrhage and Thrombocytopenia After AstraZeneca COVID-19 Vaccine: Clinical and Diagnostic Challenges of Vaccine-Induced Thrombotic Thrombocytopenia. Cureus 2021, 13, e17637.
- Nicolson, P.L.; Montague, S.J.; Smith, C.W.; Lodwick, C.S.; Stoneley, C.; Roberts, M.; Watson, S.P.; Lowe, G.C.; Lester, W.A. Anti-PF4 levels of patients with VITT do not reduce 4 months following AZD1222 vaccination. medRxiv 2021.
- Thaler, J.; Jilma, P.; Samadi, N.; Roitner, F.; Mikušková, E.; Kudrnovsky-Moser, S.; Rettl, J.; Preiss, R.; Quehenberger, P.; Pabinger, I.; et al. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thromb. Res. 2021, 207, 126–130.
- Lacy, J.; Pavord, S.; Brown, K.E. VITT and Second Doses of Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 95.




