Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is frequently complicated by thrombosis. In some cases of severe COVID-19, fibrinolysis may be markedly enhanced within a few days, resulting in fatal bleeding. In the treatment of COVID-19, attention should be paid to both coagulation activation and fibrinolytic activation. Various thromboses are known to occur after vaccination with SARS-CoV-2 vaccines. Vaccine-induced immune thrombotic thrombocytopenia (VITT) can occur after adenovirus-vectored vaccination, and is characterized by the detection of anti-platelet factor 4 antibodies by enzyme-linked immunosorbent assay and thrombosis in unusual locations such as cerebral venous sinuses and visceral veins. Treatment comprises high-dose immunoglobulin, argatroban, and fondaparinux. Some VITT cases show marked decreases in fibrinogen and platelets and marked increases in D-dimer, suggesting the presence of enhanced-fibrinolytic-type disseminated intravascular coagulation with a high risk of bleeding. In the treatment of VITT, evaluation of both coagulation activation and fibrinolytic activation is important, adjusting treatments accordingly to improve outcomes.
1. Introduction
The novel coronavirus disease 2019 (COVID-19), which was first identified in Wuhan in December 2019, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of the end of January 2022, approximately two years had passed since the beginning of the COVID-19 pandemic, with the number of infected people worldwide exceeding 3.5 billion and the number of deaths exceeding 550 million at that time. COVID-19 is often complicated by coagulopathy and thrombosis. Though immobility of severely ill patients needs to be considered [
1], the incidence of thrombosis appears high among severe COVID-19 cases [
2,
3].
SARS-CoV-2 vaccines have been developed by many countries to prevent the spread of the infection and reduce the severity of COVID-19. However, it is known that vaccination rarely causes coagulopathy [
4].
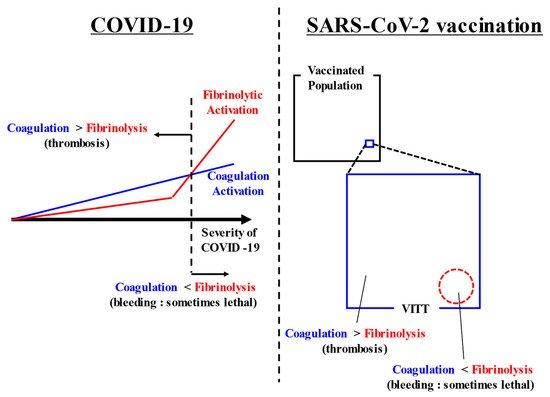
In both COVID-19-associated coagulopathy and SARS-CoV-2 vaccination-associated coagulopathy, attention to both thrombosis and bleeding is required. While much attention has been given to thrombosis and coagulation activation, almost never have reports discussed bleeding and fibrinolysis. We would therefore like to highlight and discuss these relatively neglected factors. A summarizing figure is shown in (Figure 1).
Figure 1. Summarizing figure. COVID-19 causes coagulation activation depending on its severity. In addition, some cases of severe COVID-19 have markedly increased fibrinolysis. Thrombosis appears as the main symptom when coagulation activation exceeds fibrinolytic activation. Conversely, bleeding appears as the main symptom when fibrinolytic activation exceeds coagulation activation. After SARS-CoV-2 vaccination, VITT rarely occurs. In most cases, coagulation activation exceeds fibrinolytic activation and thrombosis occurs, but in some cases, bleeding appears when fibrinolytic activation exceeds coagulation activation. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VITT, vaccine-induced immune thrombotic thrombocytopenia.
2. SARS-CoV-2 Vaccination-Associated Coagulopathy
2.1. Safety of Vaccination in Persons with Coagulation Abnormalities
Safe administration of vaccines to patients with coagulation abnormalities involves two considerations: first, whether the physical invasion associated with vaccination will cause bleeding in patients with bleeding tendencies or hemorrhagic diseases; and second, whether patients with hemorrhagic or thrombotic diseases will experience exacerbation or flare-up of the underlying disease.
2.1.1. Preventing Bleeding Due to Vaccination in Patients with Coagulation Abnormalities
Vaccination of patients on antiplatelet and anticoagulant medications (warfarin and DOAC) is certainly possible. However, adequate compression of the vaccination site should be performed after vaccination [
162]. If the PT-INR is above the therapeutic range, vaccination should be postponed until the PT-INR is back within the therapeutic range [
161], and in DOAC-treated patients, vaccination is best avoided when blood levels of DOACs are high.
Vaccination after prophylaxis treatment is recommended in patients with hemophilia who are receiving regular prophylaxis treatment and in patients with severe von Willebrand disease.
Thrombocytopenia/dysfunctional platelets do not interfere with vaccination, but adequate compression of the vaccination site after vaccination is advisable.
In chronic DIC (e.g., cases with aortic aneurysms and vascular malformations), the invasiveness of vaccination may cause severe bleeding and should be discussed with the attending physician [
128].
2.1.2. Vaccination and Thrombotic/Hemorrhagic Disease Exacerbations and Relapses
In patients with immune thrombocytopenia (ITP), the relapse of ITP with vaccination represents a matter of concern. In fact, vaccination of patients with ITP resulted in a ≧20% decrease in platelet count from baseline in about half of patients [
163]. However, no reports have suggested that vaccination should be withdrawn in patients with ITP. Risk factors for ITP relapse with vaccination include ongoing treatment, old age [
164], and post-splenectomy status or a long history of prior treatment [
165]. In terms of platelets, low platelet counts are reportedly associated with a higher risk of relapse [
164], and platelet counts often decrease rapidly in patients with normal platelet count [
166]. Regardless of the platelet count, attention should be paid to transition of the platelet count in ITP patients. The nadir of platelet counts in ITP patients has been observed around 7–10 days after vaccination [
165]. Reports have also recommended measuring platelet counts on days 3–7 after vaccination to confirm the presence or absence of ITP flare-up [
167]. Some reports have described ITP cases after vaccination in which the patients were able to receive additional vaccinations without problems [
168].
Furthermore, a decrease in platelet count greater than 50% from baseline was observed in 1.0% of healthy controls [
164], suggesting that a significant number of patients after vaccination may experience de novo ITP, including cases in which clinical symptoms do not develop and go unnoticed [
164].
In patients with paroxysmal nocturnal hemoglobinuria (PNH) or atypical hemolytic uremic syndrome using eculizumab or ravulizumab, vaccination within one week of eculizumab administration and within four weeks of ravulizumab administration is recommended to maintain blood levels of these agents [
169]. Thus far, no reports have described an increased risk of relapse of the primary disease after vaccination in specific diseases or a need to refrain from vaccination, and the benefits of vaccination are considered to outweigh the disadvantages. Adequate consultation with the patient and careful observation of changes in coagulation studies are important.
2.2. Novel Clotting Abnormalities after Vaccination
Vaccines are being developed and marketed around the world by a number of manufacturers. The number of vaccinations worldwide has exceeded 10 billion, and more than 50% of the global population had completed the required number of vaccinations as of the end of January 2022 [
170]. However, the occurrence of thrombosis and bleeding after vaccination remains problematic.
2.2.1. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
A series of reports have described thrombosis with thrombocytopenia after vaccination with the adenovirus-vectored vaccine (ChAdOx1 nCoV-19) [
171,
172,
173,
174]. Similar reports have been made for Ad26.COV2.S, which is an adenovirus vector type vaccine [
175,
176]. Thrombi occurring after adenovirus-vectored vaccinations have also been characterized as occurring in unusual sites, such as cerebral venous sinuses and visceral veins (e.g., portal vein) [
171].
Thrombosis with thrombocytopenia following administration of adenovirus vector vaccines has been referred to as VITT, as well as vaccine-induced prothrombotic immune thrombocytopenia and thrombosis with thrombocytopenia syndrome [
177]. VITT is currently the most commonly used term and is considered by the authors as the most appropriate term for describing this condition.
2.2.2. Thrombotic/Bleeding Disorders Other Than VITT
In addition to the adenovirus vector vaccines, ChAdOx1-nCoV-19 and Ad26.COV2.S, the mRNA vaccines, BNT162b2 and mRNA-1273, have been reported to cause various thrombotic/hemorrhagic diseases. Thrombotic/hemorrhagic adverse effects of SARS-CoV-2 vaccines, including those of the adenovirus vector type and the mRNA type, are described below. The diseases that should be differentiated from VITT are shown in Table 3.
Table 3. Diseases that should be differentiated from VITT and their key considerations.
| Disease Name |
Abbreviation |
Important Clinical and Laboratory Findings |
| Heparin-induced thrombocytopenia |
HIT |
History of exposure to heparin, 4T’s score |
| Thrombotic microangiopathy |
TMA |
Appearance of schizocytes (peripheral blood smear), marked decrease in haptoglobin |
| Thrombotic thrombocytopenic purpura |
TTP |
A type of TMA with markedly reduced ADAMTS13 activity with ADAMTS13 inhibitor |
| Immune thrombocytopenia |
ITP |
Diagnosis of exclusion. Increased megakaryocytes in bone marrow and positive antiplatelet antibodies assist in diagnosis |
| Antiphospholipid antibody syndrome |
APS |
Positive for at least one of the following antibodies: lupus anticoagulant; anticardiolipin antibody; and anti-β2 GPI antibody |
| Paroxysmal nocturnal hemoglobinuria |
PNH |
Hemolysis (normocytic anemia, elevated reticulocyte, elevated indirect bilirubin, elevated LDH, decreased haptoglobin),
presence of PNH type-cells (CD55/59-negative) |
| Disseminated intravascular coagulation |
DIC |
PT, APTT, fibrinogen, FDP, D-dimer, AT, TAT, PIC, plasminogen, α2PI |
- (1)
-
Hemorrhagic disease
Complications such as hematuria, extensive petechial hemorrhage, subarachnoid hemorrhage [
178], immune thrombocytopenia [
179,
180,
181,
182,
183], Evans syndrome [
184], acquired hemophilia [
185], and factor XIII inhibitor [
186] have been reported with the use of adenovirus vector and mRNA vaccines. In particular, when platelet transfusion is performed for hemorrhagic disease, examination to rule out VITT should always be performed, because with the presence of VITT in the background, the underlying condition may be aggravated after platelet transfusion.
Since thrombosis after vaccination initially drew worldwide attention, there is a tendency to focus on thrombosis, but it is important to remember that hemorrhagic side effects may also occur.
- (2)
-
Thrombotic disease
Reports have described carotid artery thrombosis and aortic thrombotic complications [
187], deep vein thrombosis [
188,
189], myocardial infarction [
190], adrenal infarction/hemorrhage [
191,
192], and cerebral infarction/intracranial hemorrhage [
193] following adenovirus vector and mRNA vaccinations. Examination to rule out VITT should always be conducted, not only in the case of hemorrhagic events, but also in the case of thrombotic events, due to the concern that a background of VITT may exacerbate the underlying condition with heparin administration.
- (3)
-
Thrombophilia with low platelet count
Numerous reports have described thrombotic thrombocytopenic purpura [
194,
195,
196] and DIC [
197]. The exclusion of VITT is still important to determine an accurate treatment strategy.
According to a systematic review of cardiovascular and hematological events after SARS-CoV-2 vaccination, these abnormalities tended to be slightly more frequent among women and young people. Adenovirus-vectored vaccines, ChAdOx1-nCoV-19 and for Ad26.COV2.S, were associated with higher rates of thrombosis and thrombocytopenia, while mRNA vaccines, BNT162b2 and mRNA-1273, were associated with higher rates of cardiac injury in a higher proportion of cases [
198]. Although accurate evaluation is difficult due to differences in the number of vaccinations, age group, race, and other factors among manufacturers, certain tendencies in thrombotic/hemorrhagic adverse effects may exist depending on the type of vaccine. Although this remains a subject for further study, knowing which manufacturer’s vaccine a patient received is still important in actual clinical practice.
3.3. Pathophysiology of VITT
VITT is characterized by thrombosis occurring at unusual sites such as cerebral venous sinuses and visceral veins (thrombosis in common sites such as venous thromboembolism is also seen), decreased platelet count, and coagulation abnormalities (e.g., elevated D-dimer) occurring 4–28 days after vaccination. In addition, a positive (usually strongly positive) result from enzyme-linked immunosorbent assay (ELISA) for anti-platelet factor 4 (PF4) antibodies is the basis for a definitive diagnosis, even though heparin is not used. As such, the condition of VITT is considered similar to immune (spontaneous) heparin-induced thrombocytopenia (HIT) [
199]. An important point is that measurement of anti-PF4 antibodies by chemiluminescent immunoassay (CLIA) or latex agglutination will result in false-negative results, rendering definitive diagnosis impossible with these two methods [
177,
200]. Importantly, anti-PF4 antibodies should be measured by ELISA. However, false-negative results with the CLIA and latex agglutination methods can also be used as one basis for VITT diagnosis [
201].
Although the frequency of VITT is extremely low, ranging from a few to ten cases per million [
202], it is important to note that this condition is often fatal when present.
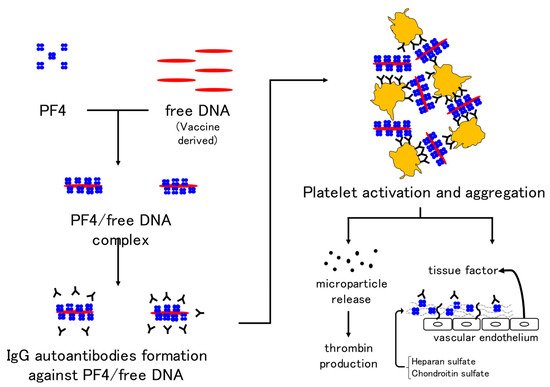
The mechanism of VITT is thought to be the formation of autoantibodies against the complex of PF4 and free DNA or the coating protein of adenoviruses and binding of the Fc portion of autoantibodies to the Fc receptor on the platelet membrane, which may induce platelet activation and aggregation. In addition, prothrombotic microparticles are released from activated platelets and thrombin production is promoted. PF4 binds to heparin sulfate and chondroitin sulfate on vascular endothelial cells, and binding of autoantibodies to these sites activates endothelial cells. Tissue factor expression from endothelial cells is also thought to activate coagulation [
171] (
Figure 3). Although 67% (29/43) of healthy controls are positive for anti-PF4 antibodies on day 22 after the first dose of ChAdOx1-nCoV-19, no positive patients showed high antibody titers or developed VITT [
203]. When evaluating the anti-PF4 antibody (by ELISA), attention must be paid not only to the positive or negative result, but also to the antibody titer. The prevalence of anti-PF4 antibodies is 1.0–6.6% [
204,
205] in pre-vaccinated populations. A significant number of post-vaccination anti-PF4 antibody-positive individuals are likely to have had antibodies prior to vaccination, making routine anti-PF4 antibody testing of asymptomatic individuals less useful [
206].
Figure 3. Mechanism of VITT development in adenovirus vector vaccines. Immunoglobulin G autoantibodies against the complex of platelet factor 4 and free DNA in the vaccine result in platelet activation and aggregation. In addition, the release of microparticles and activation of coagulation by vascular endothelial cells are thought to be mechanisms underlying thrombus formation in VITT. Abbreviations: VITT, vaccine-induced immune thrombotic thrombocytopenia; PF4, platelet factor 4.
Post-vaccination cerebral venous sinus thrombosis has also been reported with BNT162b2 [
207,
208,
209]. However, in all such cases, platelet counts were normal and anti-PF4 antibodies were not detected, therefore, VITT is not a consideration.
The mRNA vaccines work by delivering mRNA (for the formation of spike proteins) protected by lipid nanoparticles to ribosomes in human cells, causing the production of spike proteins [
210]. S protein produced by mRNA vaccine is not exactly the same as the S protein of SARS-CoV-2. For example, uridine of mRNA is converted to pseudouridine [
211,
212] for more efficiency and safety [
213]. The mechanism of mRNA vaccine-induced thrombosis may resemble that of COVID-19 [
209], although the details are as yet unknown.
3.4. Diagnosis of VITT
If a patient presents with any of the symptoms listed in
Table 4 within 4–28 days after vaccination (day 0 being the date of vaccination), VITT should be suspected because of the possibility of new thrombosis. Coagulation tests for PT, APTT, fibrinogen, and D-dimer (FDP) are mandatory, and PT and APTT alone are insufficient. Since some VITT patients present with DIC and bleeding tendency, measuring TAT (a marker of coagulation activation) and PIC, plasminogen, and α2PI (as markers of fibrinolysis activation) at the same time is advisable [
214].
Table 4. Clinical features indicative of VITT.
| Clinical Findings |
| (1) Onset 4–28 days after vaccination (counting the day of vaccination as day 0) |
| (2) Symptoms suggestive of stroke (unilateral facial palsy, unilateral motor palsy, language disorder, joint parallax, hemispheric neglect, etc.) |
| (3) Symptoms suggestive of cerebral venous sinus thrombosis (persistent headache, visual disturbance, seizure, nausea and vomiting, psychiatric symptoms, etc.) |
| (4) Symptoms suggestive of visceral vein thrombosis (persistent abdominal pain, nausea and vomiting, etc.) |
| (5) Symptoms suggestive of deep vein thrombosis or pulmonary thromboembolism (pain and swelling in lower limbs, chest and back pain, shortness of breath, etc.) |
| (6) Hemorrhagic tendencies such as hemorrhagic infarction, petechial hemorrhage, and mottled hemorrhage can also occur. |
As mentioned above, measurement of anti-PF4 antibodies by ELISA is of paramount importance, as almost all cases of VITT are positive and show a high titer. However, if VITT is strongly suspected, waiting for the test results may take too long, and initiation of empirical treatment is advisable.
A marked increase in D-dimer to more than four times the upper limit of normal alongside a decreased platelet count is highly suggestive of VITT, but a marked increase in D-dimer is not essential for diagnosis. This is because some VITT patients show only mild elevations in D-dimer.
Another report proposed a “pre-VITT syndrome” in which severe headache at 5–20 days after adenovirus-vectored vaccination should be considered a sign of cerebral venous sinus thrombosis [
218]. In fact, although no thrombosis was found on imaging, VITT was suspected early based on clinical symptoms and blood test findings (such as thrombocytopenia and elevated D-dimer), and treatment for VITT was started early, resulting in discharge from hospital in less than one week [
219,
220]. Notably, VITT is known to occur after administration of adenovirus vector-type COVID-19 vaccines. However, on day 10 after inoculation with the Gardasil 9 vaccine against human papillomavirus (inactivated vaccine), thrombocytopenia similar to that in VITT, venous thrombosis, elevated D-dimer, and the presence of anti-PF4-polyanion complex antibody have been reported [
221]. Similar pathologies may have been observed with other vaccinations, but have simply gone unnoticed until now.
Initiating treatment is important when VITT is suspected, as the mortality rate for VITT was an extremely high 50% when the disease was first recognized, although this has now reportedly improved to 22% and even 5% [
215]. This is probably due to increased awareness of the disease and earlier detection. The FAPIC score has been devised as a prognostic score looking at fibrinogen, age, platelet count, presence of intracerebral hemorrhage, and presence of cerebral venous sinus thrombosis [
222]: fibrinogen < 150 mg/dL; age ≤ 60 years; platelets < 25,000/μL, intracerebral hemorrhage, and cerebral venous sinus thrombosis all represent prognostic factors for poor clinical outcome. This is a simple scoring system, but does not address treatment options for each stratified risk level. In addition, fibrinogen and platelets are suspected to be involved in enhanced-fibrinolytic-type DIC, as described below.
3.5. Treatment of VITT (Attention to Fibrinolytic Pathophysiology)
The treatment strategy is based on the treatment of HIT, and includes discontinuation of heparin (although heparin may reportedly be used if HIT can be reliably excluded [
215]), high-dose immunoglobulin therapy, anticoagulants other than heparin (argatroban, fondaparinux, DOACs, etc.), and steroids have all been mentioned [
223,
224].
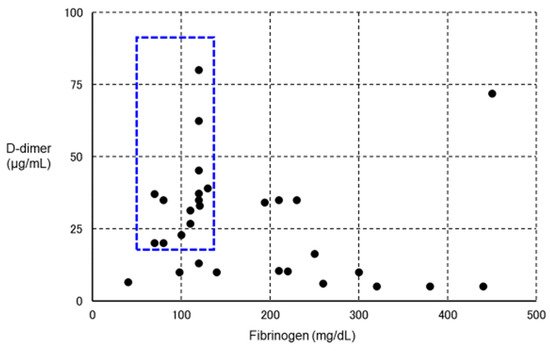
However, in some VITT patients, in addition to a marked increase in D-dimer, marked decreases in platelet count and fibrinogen have been observed (
Figure 4) [
171,
225], suggesting a change in coagulation markers as in enhanced-fibrinolytic-type DIC [
93]. If such patients are reflexively treated with anticoagulant therapy, major bleeding may result, and rigorous evaluation of coagulation and fibrinolytic markers is thus necessary. In fact, a clinical trial of argatroban for DIC was conducted in Japan more than 30 years ago, but was discontinued after the occurrence of bleeding side effects in many patients. In retrospect, we believe that argatroban was more likely to cause bleeding in patients with enhanced-fibrinolytic-type DIC, and should not be prescribed without caution in patients with enhanced-fibrinolytic-type DIC. The characteristics of suppressed-fibrinolytic-type DIC, enhanced-fibrinolytic-type DIC, and VITT are summarized in
Table 5.
Figure 4. Relationship between VITT and fibrinolytic pathophysiology. We plotted fibrinogen on the horizontal axis and D-dimer on the vertical axis for 30 definitive VITT cases (using data from Reference [
222]) in which both fibrinogen and D-dimer values were known. The lower the level of fibrinogen, the higher the level of D-dimer. In particular, patients with blue dotted squares showed a decrease in fibrinogen and a marked increase in D-dimer, suggesting the complication of enhanced-fibrinolytic-type DIC (a subject for further investigation). If anticoagulants are administered to the same intensity as in other cases, the risk of bleeding is considered high. To confirm the diagnosis of enhanced-fibrinolytic-type DIC, measurement of not only the coagulation activation marker TAT, but also the fibrinolysis activation marker PIC is essential. Patients with markedly decreased α
2PI are at high risk of bleeding. Importantly, in enhanced-fibrinolytic-type DIC, the increase in FDP is more prominent than the increase in D-dimer (discrepancy between FDP and D-dimer). Antithrombin, as a coagulation inhibitor, does not often decrease, even in patients with a marked decrease in α2PI, a fibrinolytic inhibitor (except in patients with reduced hepatic reserve). Abbreviations: VITT, vaccine-induced immune thrombotic thrombocytopenia; DIC, disseminated intravascular coagulation; TAT, thrombin–antithrombin complex; PIC, plasmin–α
2 plasmin inhibitor complex; α
2 PI, α
2 plasmin inhibitor; FDP, fibrin/fibrinogen degradation products.
Table 5. Laboratory findings in typical cases of suppressed/enhanced-fibrinolytic-type DIC and VITT.
| Disease |
Suppressed-Fibrinolytic-Type DIC |
Enhanced-Fibrinolytic-Type DIC |
VITT |
| Underlying disease/cause |
Severe sepsis |
APL, aortic aneurysm, prostate cancer, etc. |
Adenovirus vector type
vaccination |
| Pathophysiology |
Activation of coagulation and mild fibrinolysis activation |
Activation of coagulation and enhanced fibrinolysis |
Antibodies against PF4 are mediated
platelet and coagulation activation |
| Main symptom |
Organ damage |
Bleeding |
Headache, abdominal pain, etc. |
| Examination findings |
Platelet count |
Decreased |
Decreased |
Decreased |
| PT |
Prolonged |
Normal to prolonged |
Normal to prolonged * |
| APTT |
Prolonged |
Slightly shortened to prolonged |
Normal to prolonged * |
| Fibrinogen |
Normal to elevated |
Decreased |
Significantly reduced to normal |
| D-dimer |
Increased |
Increased |
Increased |
| FDP |
Increased |
Markedly increased |
Increased—markedly increased * |
| TAT |
Increased |
Increased |
Increased * |
| PIC |
Slightly increased |
Markedly increased |
Increased—markedly increased * |
| Medical treatment |
Anticoagulant therapy |
Anticoagulant therapy ± antifibrinolytic therapy |
Anticoagulant therapy other than heparin, high-dose immunoglobulin therapy, etc. |
Tranexamic acid has also been reported to save lives [
226], but the use of tranexamic acid in VITT should be considered with care, given the risk of exacerbating thrombosis. Anti-PF4 antibodies persist for at least four months after the end of VITT treatment [
227], but advantages [
227] and disadvantages [
228] are seen with respect to VITT flare-ups, so careful follow-up may be necessary.
In addition, no relapse of VITT occurred after a second additional dose of vaccine was administered to 40 patients who had developed VITT after the first dose. In many cases, mRNA-type vaccines, BNT162b2 and mRNA-1273, had been administered as the second dose, suggesting that immune responses to spike proteins are not involved in the pathogenesis of VITT. From the perspective of protection against infection, a second vaccination should be administered even after the onset of VITT [
229].
Cerebral venous sinus thrombosis occurring after vaccination with BNT162b2 (but not after vaccination with ChAdOx1-nCoV-19) did not show a decreased platelet count or anti-PF4 antibodies, was not VITT, and was treated with heparins [
208]. Which manufacturer’s vaccine was administered is an important piece of information for determining the course of treatment.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063338