Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gordon Tin Chun Wong | + 1763 word(s) | 1763 | 2022-02-28 04:36:51 | | | |
| 2 | Dean Liu | -4 word(s) | 1759 | 2022-03-14 04:08:13 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1759 | 2022-03-28 04:57:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wong, G. The Complement System in the Central Nervous System. Encyclopedia. Available online: https://encyclopedia.pub/entry/20523 (accessed on 01 March 2026).
Wong G. The Complement System in the Central Nervous System. Encyclopedia. Available at: https://encyclopedia.pub/entry/20523. Accessed March 01, 2026.
Wong, Gordon. "The Complement System in the Central Nervous System" Encyclopedia, https://encyclopedia.pub/entry/20523 (accessed March 01, 2026).
Wong, G. (2022, March 13). The Complement System in the Central Nervous System. In Encyclopedia. https://encyclopedia.pub/entry/20523
Wong, Gordon. "The Complement System in the Central Nervous System." Encyclopedia. Web. 13 March, 2022.
Copy Citation
The functions of the complement system to both innate and adaptive immunity through opsonization, cell lysis, and inflammatory activities are well known. In contrast, the role of complement in the central nervous system (CNS) which extends beyond immunity, is only beginning to be recognized as important to neurodevelopment and neurodegeneration. In addition to protecting the brain against invasive pathogens, appropriate activation of the complement system is pivotal to the maintenance of normal brain function.
complement
astrocytes
microglia
neurons
neurodevelopment
neurodegeneration
neuroinflammation
1. Introduction
The complement system is foundational to the innate immune response in defending the body against invading pathogens by phagocytosis or by the activation of the adaptive immune system. In the CNS, however, the complement system protects the brain from not only pathogens but other potentially harmful stimuli such as aberrant proteins and cellular debris [1]. Findings from studies presented will show that complement components are produced by both neurons and glial cells. This local production of complement factors may be a developmental advantage as it enables a more rapid response than reliance on peripheral production and diffusion through the blood–brain barrier (BBB). Under normal circumstances, the activation of the complement system in the CNS consists of over 30 complement factors under tight regulation [2]. However, when this well-tuned regulatory machinery malfunctions, aberrant complement factors can exacerbate neurological symptoms of brain conditions and accelerate the development of aging-related or neurodegenerative diseases [3][4][5]. Emerging evidence suggests that higher levels of complement factors are present in developing and degenerating brains and perform novel functions in neurodevelopment and contribute to the pathophysiology of neurodegenerative diseases [3][6][7][8]. Therefore, an understanding of endogenous complement production and regulation in the brain can provide insights into aberrant neurodevelopment and the genesis of neurodegeneration (Table 1).
Table 1. Complement expression in the CNS and their role in neurodevelopment and neurodegeneration.
| Complement Component |
Location | Role(s) in Normal Neurodevelopment | Pathophysiological Involvement in Neurodevelopmental and Neurodegenerative Diseases |
|---|---|---|---|
| C1q | neuron [9][10][11] microglia [2] |
Synpatic pruning [9][12] | AD: mediates glial activation and promote synapse loss [13][14] |
| ASD [15], MS [16][17][18], ALS [19][20], HD [21] | |||
| C3 | astrocyte [22][23][24][25] microglia [2] neuron [10][11][26] |
Progenitor proliferation [27], neuronal migration [28], Synaptic pruning [9] |
ASD: mediates microglia synaptic pruning [29][30] |
| AD: mediates microglial synaptic engulfment, direct neuronal toxicity, and Aβ clearance [31][32][33][34][35][36][37] | |||
| MS: activates the alternative pathway, mediate microglia and synaptic engulfment [16][17][18][38][39][40] | |||
| ALS [19][20][41], PD [42][43], HD [21][44] | |||
| PNDs: related to increase microglial activation, neuronal loss, and BBB disruption [45] | |||
| C4 | neuron [10][11][23][24] | - | Schizophrenia: each C4 allele increases the risk [46][47] |
| ALS [48][49], HD [21] | |||
| C5 | astrocyte [8][50] neuron [10][11][26] |
Progenitor proliferation [51], neuronal migration [52] |
AD: mediates pro-inflammatory responses [53][54][55] |
| ALS: mediates pro-inflammatory responses [56][57][58][59] | |||
| ASD [29] | |||
| MAC, C5-C9 | astrocyte [50] neuron [10][11][26] |
- | MS [60], ALS [49] |
| CR3 | microglia [8][24] | Synaptic pruning [61][62] | AD: mediates microglial synaptic engulfment [32][37][63] PD: mediate microglial activation [64] |
| CR4 | microglia [8][24] | - | - |
| C3aR | microglia [8][24] neuron [65][66] asotrycte [67] |
Progenitor proliferation [27], neuronal migration [52] |
AD:mediate microglial synaptic engulfment [35][68] |
| C5aR | microglia [8][24][69] neuron [65][70] astrocyte [71] |
Progenitor proliferation [51], neuronal migration [52] |
AD: mediates pro-inflammatory response [53][54] |
| ALS: recruits immune cells including peripheral cell infiltration [56][57][58][59] | |||
| MS: mediates pro-inflammatory response [60][72] | |||
| Crry | - | - | AD: anti-C3 inhibition and promotes Aβ plague formation [73] |
| MS: anti-C3 inhibition and prevents synapse loss [38] |
|||
| C1INH | astrocyte [24] neuron [2][24] |
Neuronal migration [52] | MS [16] |
| MASP1, 2 | - | Neuronal migration [28] | - |
| Factor H | astrocyte [24] microglia [74][75] |
- | AD [76] |
| Factor B | astrocyte [22][24] | - | ALS [77] |
| Factor I | asotrycte [8] | - | - |
| C4BP | astrocyte [8] | - | - |
| CD55 | astrocyte [8][66] neuron [2][24] |
- | ALS [77] |
| CD59 | asotrycte [8][66] neuron [2][24] |
- | ALS [77] |
MAC: membrane attack complex; AD: Alzheimer’s disease; ALS: amyotrophic lateral sclerosis; MS: multiple sclerosis; PD: Parkinson’s disease; HD: Huntington’s disease; PNDs: perioperative neurocognitive disorders.
2. Complement Component and Pathways
There are three distinct pathways that activate the complement system: the classical, lectin, and alternative pathways (Figure 1). The classical pathway is initiated by C1q complex formation when C1q recognizes antigen–antibody complex [78]. The lectin pathway is analogous to the classical pathway, which begins from the binding of mannose residues by mannose-binding lectin (MBL). C1q complex and mannose-binding lectin associated proteases (MASPs) cleave C2 and C4 into C2a and C4b, followed by C3 convertase (C4b–C2a) formation [79][80]. The alternative pathway can be initiated from C3 hydrolysis or from C3b directly. Once factor B binds with C3(H2O) or C3b, it is cleaved by factor D to become C3(H2O)Bb or C3b-Bb as another C3 convertase, which further produces more C3b to amplify the complement cascade [81]. Thus, the alternative pathway accounts for around 80–90% of complement activation [9][10][12][13]. C3 convertases cleave C3 into C3a and C3b; C3a can induce downstream activation by binding to C3aR, and C3b binds to CR3 or CR4 to mediate phagocytosis [82]. C3b can further combine with C4b–C2a and C3b-Bb to generate C5 convertases (C4b–C2a–C3b and C3b–Bb–C3b) and activate the terminal pathway which is regarded as the effector pathway of the complement system [6][10][11][13]. Similar to C3, C5 convertases cleave C5 into C5a and C5b; C5a is another important chemotactic protein similar to C3a, and C5b assembles with C6–9 to generate the membrane attack complex (MAC) [79][80].
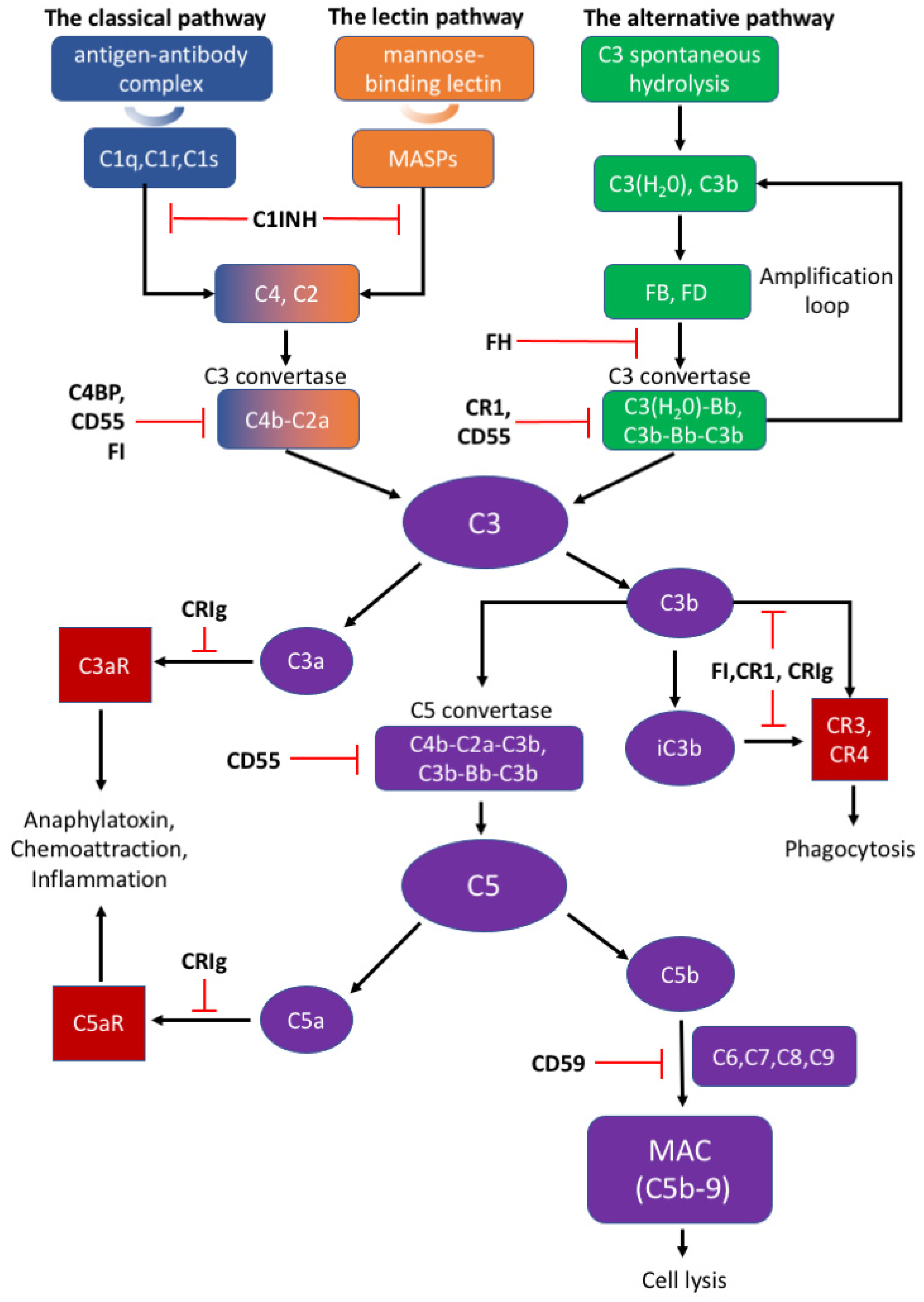
Figure 1. The activation and regulation of the complement system.
The appropriate activation of the complement cascades rely on a series of tightly regulated soluble and membrane-bound complement inhibitors. Such complement inhibitors have been shown to successfully control complement activation in animal models with CNS disorders [4]. Soluble complement inhibitors include C1 inhibitor (C1INH), complement inhibitor C4b binding protein (C4BP), factor H (FH) and factor I (FI) while complement receptor type 1 (CR1), CRIg, CD55, and CD59 belong to the membrane-bound complement inhibitors [83]. C1INH, also known as SERPING1, inactivates the proteolytic effects of the C1 complex, MASP1, and MASP2, to inhibit the classical and lectin pathways [79][84]. Deficiency of C1INH can affect normal neurodevelopment, indicating that appropriate complement system function is important to the CNS [1]. C4BP can bind to C4b and act as a decay-accelerating factor [83]. Moreover, FH is the dominant regulatory factor of the alternative pathway and by competitively binding to C3b, it destabilizes C3 and C5 convertase both on cell surfaces and in plasma [79]. FI can permanently inactivate C3b and iC3b and degrade C3 convertase by cleaving the C4b component aside by cofactors such as FH, C1INH, and C4BP [79]. CR1 is a single-pass membrane glycoprotein expressed on different cells and acts as a cofactor to FI in accelerating the dissociation of C3 convertase [79][81]. Rodents bear only the complement receptor type-1 related protein (Crry) in place of CR1 [85]. Another potent inhibitory factor CRIg converts C3a or C5a into inactive forms, which impairs signal transmission through the C3a or C5a receptors [79]. An inactive C3b product (iC3b) also interacts with CR1 or CRIg for further degradation [78][79]. CD55 is widely expressed on various cells and inhibits the formation of and accelerates the dissociation of C3 convertases among all three complement pathways [78][83]. Different from other complement inhibitors, CD59 binds to C5b-8 on the host cell surface and blocks the binding and polymerization of C9 to prevent MAC formation [79].
It is well-known that 90% of soluble serum complement components are derived from the liver constitutively and activated immune cells are an important source of inducible complement protein [81]. However, the CNS may not be exposed to the same composition of complement components as in the periphery due to selective restriction of the BBB [1][2][17]. Recent studies reveal that complement components could be synthesized locally by resident cells in the CNS that provide “immunosurveillance” to maintain normal functionality in the brain [86].
3. Complement System in the Developing Brain
Neurodevelopment is a complicated process involving various signaling pathways and molecular mechanisms. It has been shown that complement components are upregulated during pre- and post-natal developmental stages of the CNS, while dramatically decreasing in the matured brain. This development-dependent dynamic change is essential for appropriate development, while imbalances in the complement cascades may result in vulnerability to developmental diseases, such as schizophrenia and autism [1][2][6][7][85].
4. The Complement System in Neurodegenerative Diseases
The pattern of complement expression changes throughout life, with high levels appearing in young or aged brains, but dramatically decreases and remains relatively low and stable in healthy adult brains [63]. In addition to the complement system’s function and contribution during neurodevelopment, increased levels of complement components are also observed in neurodegenerative diseases, including AD, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Huntington’s disease (HD), and perioperative neurodegenerative disorders (PNDs). Emerging evidence suggests that the activation of complement system plays a critical role in the pathological mechanisms of these neurodegenerative diseases.
5. Therapies Targeting the Complement System
Targeting complement activation bears the therapeutic potential to minimize complement-mediated tissue damage that may occur in trauma, autoimmune diseases, neurological diseases, and neurodegenerative diseases. Currently, anti-complement agents are available which mainly inhibit convertase assembly and cleavage, MAC formation, and the C5–C5aR interaction. The clinical trials on neurological diseases mainly focus on the PNS, neuromuscular junction, and muscle. To date, agents targeting C5 provide the most successful therapeutic strategy, with complement treatment in myasthenia gravis (MG) and neuromyelitis optica spectrum disorders (NMOSD) being the most developed [85][87][88].
Table 2. Complement Therapies, Current Clinical Development and Preclinical Studies on CNS Disease.
| Therapy | Drug Class | Mechanism | Approved Clinical Trials | Preclinical Study on CNS Disease | |
|---|---|---|---|---|---|
| C1q | Anti-C1q antibody (ANX005) | Monoclonal antibody | Bind to C1q, inhibit Classical pathway | none | GBS, AD [89] |
| C1s | Sutimlimab (BIVV009) | Monoclonal antibody | Bind to C1s | CAD [90][91][92] PNH [93] |
None |
| C3 | high-dose IVIg | IgG | Unspecific, form complex with C3b, inhibit C3 convertase | Clinical trials on MG, GBS and others [87][88] MCI: [94] |
Stroke: [95][96] AD: [97][98] |
| Compstatin (APL-2 or Pegcetacoplan, AMY-101) |
cyclic peptides | Bind to C3, interfere C3 convertase function and C3 cleavage | PNH: APL-2, Phase III, compared with eculizumab [99] AMD: phase 2 [100] Periodontitis: AMY-101, phase 2 [101] |
none | |
| C5 | Eculizumab | Monoclonal antibody | Bind to C5, prevent C5 cleavage, inhibit MAC assembly | PNH: FDA-approved treatment; compared with Ravulizumab [102] MG: REGAIN, phase3 [103][104] NMOSD: PREVENT, phase3 [105][106] GBS: phase 2, compared with IVIg [107] |
none |
| Ravulizumab (ALXN1210) |
Monoclonal antibody | Bind to C5, prevent C5 cleavage, inhibit MAC assembly | PNH: FDA-approved Treatment; compared with Eculizumab [102] MG: phase 3 [108] NMOSD: phase 3 [109] |
none | |
| Tesidolumab | Monoclonal antibody | Neutralization of C5, Inhibit terminal complement activation |
PNH: phase 2 [87] | none | |
| SKY59 | Monoclonal antibody | Long-lasting Neutralization of C5 | PNH: phase1/2 [88] | none | |
| Zilucoplan | peptide | prevents the cleavage of C5 into C5a and C5b | MG: phase 2 [110] | none | |
| Cemdisiran | RNAi | Suppress C5 production | PNH: pharmacological study [111] | none | |
| C5aR | PMX53 | cyclic hexapeptides | C5aR1 antagonists | none | I/R injury: [112] |
| PMX205 | cyclic hexapeptides | C5aR1 antagonists | none | AD: [54][69] ALS: [57][113] |
CAD: cold agglutinin disease; PNH: Paroxysmal Nocturnal Hemoglobinuria; MG: myasthenia gravis; GBS: Guillain-Barré syndrome; MCI: mild cognitive impairment; AMD: Age-Related Macular Degeneration; NMOSD: neuromyelitis optica spectrum disorders; I/R injury: ischemia/reperfusion injury.
References
- Lee, J.D.; Coulthard, L.G.; Woodruff, T.M. Complement dysregulation in the central nervous system during development and disease. Semin. Immunol. 2019, 45, 101340.
- Veerhuis, R.; Nielsen, H.M.; Tenner, A.J. Complement in the brain. Mol. Immunol. 2011, 48, 1592–1603.
- Propson, N.E.; Gedam, M.; Zheng, H. Complement in Neurologic Disease. Annu. Rev. Pathol. 2021, 16, 277–298.
- Hammad, A.; Westacott, L.; Zaben, M. The role of the complement system in traumatic brain injury: A review. J. Neuroinflamm. 2018, 15, 24.
- Ziabska, K.; Ziemka-Nalecz, M.; Pawelec, P.; Sypecka, J.; Zalewska, T. Aberrant Complement System Activation in Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4675.
- Stephan, A.; Barres, B.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning During Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369.
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. Complement: The Emerging Architect of the Developing Brain. Trends Neurosci. 2018, 41, 373–384.
- Barnum, S.R. Complement biosynthesis in the central nervous system. Crit. Rev. Oral. Biol. Med. 1995, 6, 132–146.
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178.
- Shen, Y.; Li, R.; McGeer, E.G.; McGeer, P.L. Neuronal expression of mRNAs for complement proteins of the classical pathway in Alzheimer brain. Brain Res. 1997, 769, 391–395.
- Thomas, A.; Gasque, P.; Vaudry, D.; Gonzalez, B.; Fontaine, M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int. Immunol. 2000, 12, 1015–1023.
- Bialas, A.R.; Stevens, B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 2013, 16, 1773–1782.
- Fonseca, M.I.; Zhou, J.; Botto, M.; Tenner, A.J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 2004, 24, 6457–6465.
- Dejanovic, B.; Huntley, M.A.; De Mazière, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e7.
- Corbett, B.A.; Kantor, A.B.; Schulman, H.; Walker, W.L.; Lit, L.; Ashwood, P.; Rocke, D.M.; Sharp, F.R. A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol. Psychiatry 2007, 12, 292–306.
- Ingram, G.; Loveless, S.; Howell, O.W.; Hakobyan, S.; Dancey, B.; Harris, C.L.; Robertson, N.P.; Neal, J.W.; Morgan, B.P. Complement activation in multiple sclerosis plaques: An immunohistochemical analysis. Acta Neuropathol. Commun. 2014, 2, 53.
- Michailidou, I.; Willems, J.G.; Kooi, E.J.; van Eden, C.; Gold, S.M.; Geurts, J.J.; Baas, F.; Huitinga, I.; Ramaglia, V. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 2015, 77, 1007–1026.
- Watkins, L.M.; Neal, J.W.; Loveless, S.; Michailidou, I.; Ramaglia, V.; Rees, M.I.; Reynolds, R.; Robertson, N.P.; Morgan, B.P.; Howell, O.W. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J. Neuroinflamm. 2016, 13, 161.
- Heurich, B.; El Idrissi, N.B.; Donev, R.M.; Petri, S.; Claus, P.; Neal, J.; Morgan, B.P.; Ramaglia, V. Complement upregulation and activation on motor neurons and neuromuscular junction in the SOD1 G93A mouse model of familial amyotrophic lateral sclerosis. J. Neuroimmunol. 2011, 235, 104–109.
- Bahia El Idrissi, N.; Bosch, S.; Ramaglia, V.; Aronica, E.; Baas, F.; Troost, D. Complement activation at the motor end-plates in amyotrophic lateral sclerosis. J. Neuroinflamm. 2016, 13, 72.
- Singhrao, S.K.; Neal, J.W.; Morgan, B.P.; Gasque, P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp. Neurol. 1999, 159, 362–376.
- Lévi-Strauss, M.; Mallat, M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J. Immunol. 1987, 139, 2361–2366.
- Gasque, P.; Ischenko, A.; Legoedec, J.; Mauger, C.; Schouft, M.T.; Fontaine, M. Expression of the complement classical pathway by human glioma in culture. A model for complement expression by nerve cells. J. Biol. Chem. 1993, 268, 25068–25074.
- Morgan, B.P.; Gasque, P. Expression of complement in the brain: Role in health and disease. Immunol. Today 1996, 17, 461–466.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487.
- Yu, J.X.; Bradt, B.M.; Cooper, N.R. Constitutive expression of proinflammatory complement components by subsets of neurons in the central nervous system. J. Neuroimmunol. 2002, 123, 91–101.
- Coulthard, L.G.; Hawksworth, O.A.; Conroy, J.; Lee, J.D.; Woodruff, T.M. Complement C3a receptor modulates embryonic neural progenitor cell proliferation and cognitive performance. Mol. Immunol. 2018, 101, 176–181.
- Gorelik, A.; Sapir, T.; Haffner-Krausz, R.; Olender, T.; Woodruff, T.M.; Reiner, O. Developmental activities of the complement pathway in migrating neurons. Nat. Commun. 2017, 8, 15096.
- Fagan, K.; Crider, A.; Ahmed, A.O.; Pillai, A. Complement C3 Expression Is Decreased in Autism Spectrum Disorder Subjects and Contributes to Behavioral Deficits in Rodents. Mol. Neuropsychiatry 2017, 3, 19–27.
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 2018, 9, 3769.
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 2015, 85, 101–115.
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716.
- Shi, Q.; Chowdhury, S.; Ma, R.; Le, K.X.; Hong, S.; Caldarone, B.J.; Stevens, B.; Lemere, C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 2017, 9, eaaf6295.
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018, 83, 544–552.
- Litvinchuk, A.; Wan, Y.-W.; Swartzlander, D.B.; Chen, F.; Cole, A.; Propson, N.E.; Wang, Q.; Zhang, B.; Liu, Z.; Zheng, H. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer’s Disease. Neuron 2018, 100, 1337–1353.e5.
- Wu, T.; Dejanovic, B.; Gandham, V.D.; Gogineni, A.; Edmonds, R.; Schauer, S.; Srinivasan, K.; Huntley, M.A.; Wang, Y.; Wang, T.M.; et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019, 28, 2111–2123.e6.
- Fu, H.; Liu, B.; Frost, J.L.; Hong, S.; Jin, M.; Ostaszewski, B.; Shankar, G.M.; Costantino, I.M.; Carroll, M.C.; Mayadas, T.N.; et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 2012, 60, 993–1003.
- Werneburg, S.; Jung, J.; Kunjamma, R.B.; Ha, S.K.; Luciano, N.J.; Willis, C.M.; Gao, G.; Biscola, N.P.; Havton, L.A.; Crocker, S.J.; et al. Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity 2020, 52, 167–182.e7.
- Hammond, J.W.; Bellizzi, M.J.; Ware, C.; Qiu, W.Q.; Saminathan, P.; Li, H.; Luo, S.; Ma, S.A.; Li, Y.; Gelbard, H.A. Complement-dependent synapse loss and microgliosis in a mouse model of multiple sclerosis. Brain Behav. Immun. 2020, 87, 739–750.
- Tassoni, A.; Farkhondeh, V.; Itoh, Y.; Itoh, N.; Sofroniew, M.V.; Voskuhl, R.R. The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression. Sci. Rep. 2019, 9, 10010.
- Donnenfeld, H.; Kascsak, R.J.; Bartfeld, H. Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J. Neuroimmunol. 1984, 6, 51–57.
- Brennan, F.H.; Lee, J.D.; Ruitenberg, M.J.; Woodruff, T.M. Therapeutic targeting of complement to modify disease course and improve outcomes in neurological conditions. Semin. Immunol. 2016, 28, 292–308.
- Carpanini, S.M.; Torvell, M.; Morgan, B.P. Therapeutic Inhibition of the Complement System in Diseases of the Central Nervous System. Front. Immunol. 2019, 10, 362.
- Larkin, P.B.; Muchowski, P.J. Genetic Deficiency of Complement Component 3 Does Not Alter Disease Progression in a Mouse Model of Huntington’s Disease. J. Huntingt. Dis. 2012, 1, 107–118.
- Xiong, C.; Liu, J.; Lin, D.; Zhang, J.; Terrando, N.; Wu, A. Complement activation contributes to perioperative neurocognitive disorders in mice. J. Neuroinflamm. 2018, 15, 254.
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016, 530, 177–183.
- Woo, J.J.; Pouget, J.G.; Zai, C.C.; Kennedy, J.L. The complement system in schizophrenia: Where are we now and what’s next? Mol. Psychiatry 2020, 25, 114–130.
- Kawamata, T.; Akiyama, H.; Yamada, T.; McGeer, P.L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992, 140, 691–707.
- Sta, M.; Sylva-Steenland, R.M.; Casula, M.; de Jong, J.M.; Troost, D.; Aronica, E.; Baas, F. Innate and adaptive immunity in amyotrophic lateral sclerosis: Evidence of complement activation. Neurobiol. Dis. 2011, 42, 211–220.
- Gasque, P.; Fontaine, M.; Morgan, B.P. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J. Immunol. 1995, 154, 4726–4733.
- Coulthard, L.G.; Hawksworth, O.A.; Li, R.; Balachandran, A.; Lee, J.D.; Sepehrband, F.; Kurniawan, N.; Jeanes, A.; Simmons, D.G.; Wolvetang, E.; et al. Complement C5aR1 Signaling Promotes Polarization and Proliferation of Embryonic Neural Progenitor Cells through PKCζ. J. Neurosci. 2017, 37, 5395–5407.
- Gorelik, A.; Sapir, T.; Woodruff, T.M.; Reiner, O. Serping1/C1 Inhibitor Affects Cortical Development in a Cell Autonomous and Non-cell Autonomous Manner. Front. Cell. Neurosci. 2017, 11, 169.
- Hernandez, M.X.; Jiang, S.; Cole, T.A.; Chu, S.H.; Fonseca, M.I.; Fang, M.J.; Hohsfield, L.A.; Torres, M.D.; Green, K.N.; Wetsel, R.A.; et al. Prevention of C5aR1 signaling delays microglial inflammatory polarization, favors clearance pathways and suppresses cognitive loss. Mol. Neurodegener. 2017, 12, 66.
- Fonseca, M.I.; Ager, R.R.; Chu, S.H.; Yazan, O.; Sanderson, S.D.; LaFerla, F.M.; Taylor, S.M.; Woodruff, T.M.; Tenner, A.J. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J. Immunol. 2009, 183, 1375–1383.
- Landlinger, C.; Oberleitner, L.; Gruber, P.; Noiges, B.; Yatsyk, K.; Santic, R.; Mandler, M.; Staffler, G. Active immunization against complement factor C5a: A new therapeutic approach for Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 150.
- Parker, S.E.; Hanton, A.M.; Stefanou, S.N.; Noakes, P.G.; Woodruff, T.M.; Lee, J.D. Revisiting the role of the innate immune complement system in ALS. Neurobiol. Dis. 2019, 127, 223–232.
- Lee, J.D.; Kumar, V.; Fung, J.N.; Ruitenberg, M.J.; Noakes, P.G.; Woodruff, T.M. Pharmacological inhibition of complement C5a-C5a(1) receptor signalling ameliorates disease pathology in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Br. J. Pharm. 2017, 174, 689–699.
- Lee, J.D.; Levin, S.C.; Willis, E.F.; Li, R.; Woodruff, T.M.; Noakes, P.G. Complement components are upregulated and correlate with disease progression in the TDP-43(Q331K) mouse model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2018, 15, 171.
- Wang, H.A.; Lee, J.D.; Lee, K.M.; Woodruff, T.M.; Noakes, P.G. Complement C5a-C5aR1 signalling drives skeletal muscle macrophage recruitment in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Skelet. Muscle 2017, 7, 10.
- Michailidou, I.; Jongejan, A.; Vreijling, J.P.; Georgakopoulou, T.; de Wissel, M.B.; Wolterman, R.A.; Ruizendaal, P.; Klar-Mohamad, N.; Grootemaat, A.E.; Picavet, D.I.; et al. Systemic inhibition of the membrane attack complex impedes neuroinflammation in chronic relapsing experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2018, 6, 36.
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705.
- Vukojicic, A.; Delestrée, N.; Fletcher, E.V.; Pagiazitis, J.G.; Sankaranarayanan, S.; Yednock, T.A.; Barres, B.A.; Mentis, G.Z. The Classical Complement Pathway Mediates Microglia-Dependent Remodeling of Spinal Motor Circuits during Development and in SMA. Cell Rep. 2019, 29, 3087–3100.e7.
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919.
- Hou, L.; Wang, K.; Zhang, C.; Sun, F.; Che, Y.; Zhao, X.; Zhang, D.; Li, H.; Wang, Q. Complement receptor 3 mediates NADPH oxidase activation and dopaminergic neurodegeneration through a Src-Erk-dependent pathway. Redox Biol. 2018, 14, 250–260.
- Bénard, M.; Gonzalez, B.J.; Schouft, M.-T.; Falluel-Morel, A.; Vaudry, D.; Chan, P.; Vaudry, H.; Fontaine, M. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J. Biol. Chem. 2004, 279, 43487–43496.
- Gasque, P.; Dean, Y.D.; McGreal, E.P.; VanBeek, J.; Morgan, B.P. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 2000, 49, 171–186.
- Ischenko, A.; Sayah, S.; Patte, C.; Andreev, S.; Gasque, P.; Schouft, M.T.; Vaudry, H.; Fontaine, M. Expression of a functional anaphylatoxin C3a receptor by astrocytes. J. Neurochem. 1998, 71, 2487–2496.
- Lian, H.; Litvinchuk, A.; Chiang, A.C.A.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589.
- Ager, R.R.; Fonseca, M.I.; Chu, S.-H.; Sanderson, S.D.; Taylor, S.M.; Woodruff, T.M.; Tenner, A.J. Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer’s disease. J. Neurochem. 2010, 113, 389–401.
- O’Barr, S.A.; Caguioa, J.; Gruol, D.; Perkins, G.; Ember, J.A.; Hugli, T.; Cooper, N.R. Neuronal expression of a functional receptor for the C5a complement activation fragment. J. Immunol. 2001, 166, 4154–4162.
- Gasque, P.; Singhrao, S.K.; Neal, J.W.; Götze, O.; Morgan, B.P. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am. J. Pathol. 1997, 150, 31–41.
- Loveless, S.; Neal, J.W.; Howell, O.W.; Harding, K.E.; Sarkies, P.; Evans, R.; Bevan, R.J.; Hakobyan, S.; Harris, C.L.; Robertson, N.P.; et al. Tissue microarray methodology identifies complement pathway activation and dysregulation in progressive multiple sclerosis. Brain Pathol. 2018, 28, 507–520.
- Wyss-Coray, T.; Yan, F.; Lin, A.H.; Lambris, J.D.; Alexander, J.J.; Quigg, R.J.; Masliah, E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl. Acad. Sci. USA 2002, 99, 10837–10842.
- Lukiw, W.J.; Alexandrov, P.N. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol. Neurobiol. 2012, 46, 11–19.
- Pareek, S.; Roy, S.; Kumari, B.; Jain, P.; Banerjee, A.; Vrati, S. MiR-155 induction in microglial cells suppresses Japanese encephalitis virus replication and negatively modulates innate immune responses. J. Neuroinflamm. 2014, 11, 97.
- Toledo, J.B.; Korff, A.; Shaw, L.M.; Trojanowski, J.Q.; Zhang, J. Low levels of cerebrospinal fluid complement 3 and factor H predict faster cognitive decline in mild cognitive impairment. Alzheimers Res. 2014, 6, 36.
- Lee, J.D.; Kamaruzaman, N.A.; Fung, J.N.; Taylor, S.M.; Turner, B.J.; Atkin, J.D.; Woodruff, T.M.; Noakes, P.G. Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2013, 10, 119.
- Walport, M.J. Complement. First of two parts. N Engl J Med 2001, 344, 1058–1066.
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492.
- Tegla, C.A.; Cudrici, C.; Patel, S.; Trippe, R., 3rd; Rus, V.; Niculescu, F.; Rus, H. Membrane attack by complement: The assembly and biology of terminal complement complexes. Immunol. Res. 2011, 51, 45–60.
- Dinasarapu, A.R.; Chandrasekhar, A.; Sahu, A.; Subramaniam, S. Complement C3. UCSD Signal. Gatew. Mol. Pages 2012, 15.
- Presumey, J.; Bialas, A.R.; Carroll, M.C. Complement System in Neural Synapse Elimination in Development and Disease. Adv. Immunol. 2017, 135, 53–79.
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740.
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797.
- Tenner, A.J.; Stevens, B.; Woodruff, T.M. New tricks for an ancient system: Physiological and pathological roles of complement in the CNS. Mol. Immunol. 2018, 102, 3–13.
- Francis, K.; van Beek, J.; Canova, C.; Neal, J.W.; Gasque, P. Innate immunity and brain inflammation: The key role of complement. Expert Rev. Mol. Med. 2003, 5, 1–19.
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617.
- Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019, 18, 707–729.
- Lansita, J.A.; Mease, K.M.; Qiu, H.; Yednock, T.; Sankaranarayanan, S.; Kramer, S. Nonclinical Development of ANX005: A Humanized Anti-C1q Antibody for Treatment of Autoimmune and Neurodegenerative Diseases. Int. J. Toxicol. 2017, 36, 449–462.
- Jäger, U.; D’Sa, S.; Schörgenhofer, C.; Bartko, J.; Derhaschnig, U.; Sillaber, C.; Jilma-Stohlawetz, P.; Fillitz, M.; Schenk, T.; Patou, G.; et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: A first-in-human trial. Blood 2019, 133, 893–901.
- Röth, A.; Barcellini, W.; D’Sa, S.; Miyakawa, Y.; Broome, C.M.; Michel, M.; Kuter, D.J.; Jilma, B.; Tvedt, T.H.A.; Fruebis, J.; et al. Sutimlimab in Cold Agglutinin Disease. N. Engl. J. Med. 2021, 384, 1323–1334.
- Tvedt, T.H.A.; Steien, E.; Øvrebø, B.; Haaverstad, R.; Hobbs, W.; Wardęcki, M.; Tjønnfjord, G.E.; Berentsen, S.A. Sutimlimab, an investigational C1s inhibitor, effectively prevents exacerbation of hemolytic anaemia in a patient with cold agglutinin disease undergoing major surgery. Am. J. Hematol. 2021, 97, E51–E54.
- Gavriilaki, E.; Peffault de Latour, R.; Risitano, A.M. Advancing therapeutic complement inhibition in hematologic diseases: PNH and beyond. Blood 2021.
- Kile, S.; Au, W.; Parise, C.; Rose, K.; Donnel, T.; Hankins, A.; Chan, M.; Ghassemi, A. IVIG treatment of mild cognitive impairment due to Alzheimer’s disease: A randomised double-blinded exploratory study of the effect on brain atrophy, cognition and conversion to dementia. J. Neurol. Neurosurg. Psychiatry 2017, 88, 106–112.
- Arumugam, T.V.; Tang, S.C.; Lathia, J.D.; Cheng, A.; Mughal, M.R.; Chigurupati, S.; Magnus, T.; Chan, S.L.; Jo, D.G.; Ouyang, X.; et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 14104–14109.
- Chen, X.; Arumugam, T.V.; Cheng, Y.L.; Lee, J.H.; Chigurupati, S.; Mattson, M.P.; Basta, M. Combination Therapy with Low-Dose IVIG and a C1-esterase Inhibitor Ameliorates Brain Damage and Functional Deficits in Experimental Ischemic Stroke. Neuromol. Med. 2018, 20, 63–72.
- Sudduth, T.L.; Greenstein, A.; Wilcock, D.M. Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Aβ in APP/PS1 mice along a different time course than anti-Aβ antibodies. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 9684–9692.
- Counts, S.E.; Ray, B.; Mufson, E.J.; Perez, S.E.; He, B.; Lahiri, D.K. Intravenous immunoglobulin (IVIG) treatment exerts antioxidant and neuropreservatory effects in preclinical models of Alzheimer’s disease. J. Clin. Immunol. 2014, 34 (Suppl. 1), S80–S85.
- De Castro, C.; Grossi, F.; Weitz, I.C.; Maciejewski, J.; Sharma, V.; Roman, E.; Brodsky, R.A.; Tan, L.; Di Casoli, C.; El Mehdi, D.; et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am. J. Hematol. 2020, 95, 1334–1343.
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195.
- Hasturk, H.; Hajishengallis, G.; Lambris, J.D.; Mastellos, D.C.; Yancopoulou, D. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J. Clin. Investig. 2021, 131, e152973.
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez-Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I.; et al. Ravulizumab (ALXN1210) vs. eculizumab in C5-inhibitor-experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549.
- Murai, H.; Uzawa, A.; Suzuki, Y.; Imai, T.; Shiraishi, H.; Suzuki, H.; Okumura, M.; O’Brien, F.; Wang, J.J.; Fujita, K.P.; et al. Long-term efficacy and safety of eculizumab in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the REGAIN open-label extension study. J. Neurol. Sci. 2019, 407, 116419.
- Howard, J.F., Jr.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986.
- Pittock, S.J.; Berthele, A.; Fujihara, K.; Kim, H.J.; Levy, M.; Palace, J.; Nakashima, I.; Terzi, M.; Totolyan, N.; Viswanathan, S.; et al. Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 614–625.
- Wingerchuk, D.M.; Fujihara, K.; Palace, J.; Berthele, A.; Levy, M.; Kim, H.J.; Nakashima, I.; Oreja-Guevara, C.; Wang, K.C.; Miller, L.; et al. Long-Term Safety and Efficacy of Eculizumab in Aquaporin-4 IgG-Positive NMOSD. Ann. Neurol. 2021, 89, 1088–1098.
- Misawa, S.; Kuwabara, S.; Sato, Y.; Yamaguchi, N.; Nagashima, K.; Katayama, K.; Sekiguchi, Y.; Iwai, Y.; Amino, H.; Suichi, T.; et al. Safety and efficacy of eculizumab in Guillain-Barré syndrome: A multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. 2018, 17, 519–529.
- U.S. National Library of Medicine. Safety and Efficacy Study of Ravulizumab in Adults With Generalized Myasthenia Gravis. Available online: https://clinicaltrials.gov/ct2/show/NCT03920293 (accessed on 9 December 2021).
- U.S. National Library of Medicine. An Efficacy and Safety Study of Ravulizumab in Adult Participants With NMOSD. Available online: https://clinicaltrials.gov/ct2/show/NCT04201262 (accessed on 9 December 2021).
- Howard, J.F., Jr.; Nowak, R.J.; Wolfe, G.I.; Freimer, M.L.; Vu, T.H.; Hinton, J.L.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Farzaneh-Far, R.; et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients With Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020, 77, 582–592.
- Badri, P.; Jiang, X.; Borodovsky, A.; Najafian, N.; Kim, J.; Clausen, V.A.; Goel, V.; Habtemariam, B.; Robbie, G.J. Pharmacokinetic and Pharmacodynamic Properties of Cemdisiran, an RNAi Therapeutic Targeting Complement Component 5, in Healthy Subjects and Patients with Paroxysmal Nocturnal Hemoglobinuria. Clin. Pharm. 2021, 60, 365–378.
- Shi, Y.; Jin, Y.; Li, X.; Chen, C.; Zhang, Z.; Liu, X.; Deng, Y.; Fan, X.; Wang, C. C5aR1 Mediates the Progression of Inflammatory Responses in the Brain of Rats in the Early Stage after Ischemia and Reperfusion. ACS Chem. Neurosci. 2021, 12, 3994–4006.
- Woodruff, T.M.; Costantini, K.J.; Crane, J.W.; Atkin, J.D.; Monk, P.N.; Taylor, S.M.; Noakes, P.G. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J. Immunol. 2008, 181, 8727–8734.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
3 times
(View History)
Update Date:
28 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





