
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lijun Wang | + 2278 word(s) | 2278 | 2022-02-22 03:02:13 | | | |
| 2 | Bruce Ren | + 1 word(s) | 2279 | 2022-03-11 02:00:13 | | |
Video Upload Options
Food safety issues are a worldwide concern. Pathogens, toxins, pesticides, veterinary drugs, heavy metals, and illegal additives are frequently reported to contaminate food and pose a serious threat to human health. Conventional detection methods have difficulties fulfilling the requirements for food development in a modern society. Therefore, novel rapid detection methods are urgently needed for on-site and rapid screening of massive food samples. Due to the extraordinary properties of nanozymes and aptamers, biosensors composed of both of them provide considerable advantages in analytical performances, including sensitivity, specificity, repeatability, and accuracy. They are considered a promising complementary detection method on top of conventional ones for the rapid and accurate detection of food contaminants.
1. Introduction
2. Factors Affecting the Regulation by ssDNA of Nanozyme Activity
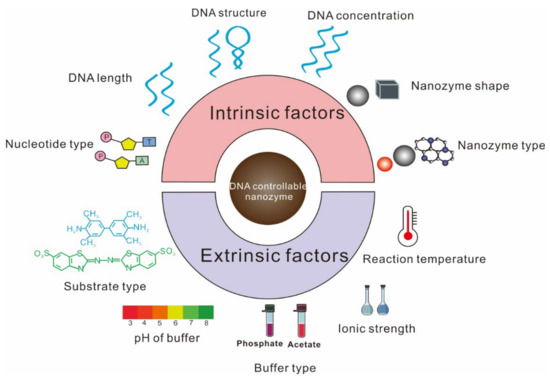
2.1. Factors Affecting ssDNA Inhibition of Nanozyme Activity
2.2. Factors Affecting ssDNA to Improve Nanozyme Activity
References
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. 2020, 62, 244–260.
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583.
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in point-of-care diagnosis: An emerging futuristic approach for biosensing. Nano-Micro Lett. 2021, 13, 193.
- Czescik, J.; Zamolo, S.; Darbre, T.; Rigo, R.; Sissi, C.; Pecina, A.; Riccardi, L.; De Vivo, M.; Mancin, F.; Scrimin, P. A gold nanoparticle nanonuclease relying on a Zn(II) mononuclear complex. Angew. Chem. Int. Ed. 2021, 60, 1423–1432.
- Nandhakumar, P.; Kim, G.; Park, S.; Kim, S.; Kim, S.; Park, J.K.; Lee, N.S.; Yoon, Y.H.; Yang, H. Metal nanozyme with ester hydrolysis activity in the presence of ammonia-borane and its use in a sensitive immunosensor. Angew. Chem. Int. Ed. 2020, 59, 22419–22422.
- Hu, X.; Huang, T.; Liao, H.; Hu, L.; Wang, M. The phosphatase-like activity of zirconium oxide nanoparticles and their application in near-infrared intracellular imaging. J. Mater. Chem. B 2020, 8, 4428–4433.
- Li, B.; Chen, D.; Nie, M.; Wang, J.; Li, Y.; Yang, Y. Carbon dots/Cu2O composite with intrinsic high protease-Like activity for hydrolysis of proteins under physiological conditions. Part. Part. Syst. Char. 2018, 35, 1800277.
- Zhang, Y.A.; Jin, Y.L.; Cui, H.X.; Yan, X.Y.; Fan, K.L. Nanozyme-based catalytic theranostics. Rsc Adv. 2020, 10, 10–20.
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. Trends Anal. Chem. 2018, 105, 218–224.
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity origin, catalytic mechanism, and biological application. Coordin. Chem. Rev. 2021, 448, 214170.
- Wang, W.Z.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. Trends Anal. Chem. 2020, 126, 115841.
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076.
- Wang, P.; Min, D.; Chen, G.; Li, M.; Tong, L.; Cao, Y. Inorganic Nanozymes: Prospects for Disease Treatments and Detection Applications. Front. Chem. 2021, 9, 773285.
- Navyatha, B.; Singh, S.; Nara, S. AuPeroxidase nanozymes promises and applications in biosensing. Biosens. Bioelectron. 2021, 175, 112882.
- Ye, M.L.; Zhu, Y.; Lu, Y.; Gan, L.; Zhang, Y.; Zhao, Y.G. Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: A review. Talanta 2021, 230, 122299.
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal- and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154.
- Jin, J.; Li, L.; Zhang, L.; Luan, Z.; Xin, S.; Song, K. Progress in the application of crbon dots-based nanozymes. Front. Chem. 2021, 9, 748044.
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237.
- Huang, X.; Zhang, S.; Tang, Y.; Zhang, X.; Bai, Y.; Pang, H. Advances in metal-organic framework-based nanozymes and their applications. Coordin. Chem. Rev. 2021, 449, 214216.
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuator B Chem. 2019, 283, 18–34.
- Wang, Q.; Liu, S.; Tang, Z. Recent progress in the design of analytical methods based on nanozymes. J. Mater. Chem. B 2021, 9, 8174–8184.
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254.
- Shen, Y.; Xu, L.; Li, Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 149–197.
- Tao, X.; Wang, X.; Liu, B.; Liu, J. Conjugation of antibodies and aptamers on nanozymes for developing biosensors. Biosens. Bioelectron. 2020, 168, 112537.
- Yan, M.M.; Chen, G.; She, Y.X.; Ma, J.; Hong, S.H.; Shao, Y.; Abd El-Aty, A.M.; Wang, M.; Wang, S.S.; Wang, J. Sensitive and simple competitive biomimetic nanozyme-linked immunosorbent assay for colorimetric and surface-enhanced raman scattering sensing of triazophos. J. Agric. Food Chem. 2019, 67, 9658–9666.
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. Trends Anal. Chem. 2018, 107, 60–77.
- Wang, L.J.; Wang, R.H.; Wei, H.; Li, Y.B. Selection of aptamers against pathogenic bacteria and their diagnostics application. World J. Microb. Biot. 2018, 34, 149.
- Dong, Y.Y.; Xu, Y.; Yong, W.; Chu, X.G.; Wang, D.N. Aptamer and its potential applications for food safety. Crit. Rev. Food Sci. 2014, 54, 1548–1561.
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-based detection methodology studies in food safety. Food Anal. Methods 2019, 12, 966–990.
- Wang, X.; Xu, Y.; Cheng, N.; Wang, X.; Huang, K.; Luo, Y. Recent advances in nucleic acid modulation for functional nanozyme. Catalysts 2021, 11, 638.
- Chang, Y.; Gao, S.; Liu, M.; Liu, J. Designing signal-on sensors by regulating nanozyme activity. Anal. Methods 2020, 12, 4708–4723.
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shuklaa, R.; Bansala, V. Aptamer-mediated ‘turn-off turn-on’ nanozyme activity of gold nanoparticles for kanamycin detection. Chem. Commun. 2014, 50, 15856–15859.
- Pautler, R.; Kelly, E.Y.; Huang, P.J.; Cao, J.; Liu, B.; Liu, J. Attaching DNA to nanoceria: Regulating oxidase activity and fluorescence quenching. ACS Appl. Mater. Interfaces 2013, 5, 6820–6825.
- Tarokh, A.; Pebdeni, A.B.; Othman, H.O.; Salehnia, F.; Hosseini, M. Sensitive colorimetric aptasensor based on g-C3N4@Cu2O composites for detection of Salmonella typhimurium in food and water. Microchim. Acta 2021, 188, 87.
- Liu, S.; Yang, M.; Guo, W. Programmable and reversible regulation of activities with DNA structures. Chem. Res. Chin. Univ. 2020, 36, 301–306.
- Weerathunge, P.; Ramanathan, R.; Torok, V.A.; Hodgson, K.; Xu, Y.; Goodacre, R.; Behera, B.K.; Bansal, V. Ultrasensitive colorimetric detection of murine norovirus using nanozyme aptasensor. Anal. Chem. 2019, 91, 3270–3276.
- Huang, L.J.; Chen, K.; Zhang, W.T.; Zhu, W.X.; Liu, X.N.; Wang, J.; Wang, R.; Hu, N.; Suo, Y.R.; Wang, J.L. ssDNA-tailorable oxidase-mimicking activity of spinel MnCo2O4 for sensitive biomolecular detection in food sample. Sens. Actuator B Chem. 2018, 269, 79–87.
- Wang, J.; Wang, J.; Zhou, P.; Tao, H.; Wang, X.; Wu, Y. Oligonucleotide-induced regulation of the oxidase-mimicking activity of octahedral Mn3O4 nanoparticles for colorimetric detection of heavy metals. Microchim. Acta 2020, 187, 99.
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim. Acta 2018, 185, 475.
- Wang, G.; Song, C.; Kong, D.; Ruan, W.; Chang, Z.; Li, Y. Two luminescent metal-organic frameworks for the sensing of nitroaromatic explosives and DNA strands. J. Mater. Chem. A 2014, 2, 2213–2220.
- Wang, L.; Liao, T.; Zhou, H.; Huang, Y.; Chen, P.; Yang, X.; Chen, X. Colorimetric method for Salmonella spp. detection based on peroxidase-like activity of Cu(II)-rGO nanoparticles and PCR. Anal. Biochem. 2021, 615, 114068.
- Kim, M.I.; Park, K.S.; Park, H.G. Ultrafast colorimetric detection of nucleic acids based on the inhibition of the oxidase activity of cerium oxide nanoparticles. Chem. Commun. 2014, 50, 9577–9580.
- Liu, B.; Liu, J. Accelerating peroxidase mimicking nanozymes using DNA. Nanoscale 2015, 7, 13831–13835.
- Song, C.; Ding, W.; Liu, H.; Zhao, W.; Yao, Y.; Yao, C. Label-free colorimetric detection of deoxyribonuclease I activity based on the DNA-enhanced peroxidase-like activity of MIL-53(Fe). New J. Chem. 2019, 43, 12776–12784.
- Lopez, A.; Zhang, Y.; Liu, J. Tuning DNA adsorption affinity and density on metal oxide and phosphate for improved arsenate detection. J. Coll. Interfaces Sci. 2017, 493, 249–256.
- Koo, K.M.; Sina, A.A.I.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. DNA-bare gold affinity interactions: Mechanism and applications in biosensing. Anal. Methods 2015, 7, 7042–7054.
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 2012, 6, 3142–3151.
- Wang, L.; Zhu, F.; Liao, S.; Chen, M.; Zhu, Y.Q.; Liu, Q.; Chen, X. Single-stranded DNA modified protonated graphitic carbon nitride nanosheets: A versatile ratiometric fluorescence platform for multiplex detection of various targets. Talanta 2019, 197, 422–430.
- Zhao, L.; Wang, J.; Su, D.; Zhang, Y.; Lu, H.; Yan, X.; Bai, J.; Gao, Y.; Lu, G. The DNA controllable peroxidase mimetic activity of MoS2 nanosheets for constructing robust colorimetric biosensor. Nanoscale 2020, 12, 19420–19428.
- Sun, S.H.; Fan, Y.F.; Du, J.Y.; Song, Z.L.; Zhao, H.M. CNT-modified MIL-88 (NH2)-Fe for enhancing DNA-regulated peroxidase-like activity. J. Anal. Test. 2019, 3, 238–245.
- Zeng, C.; Lu, N.; Wen, Y.; Liu, G.; Zhang, R.; Zhang, J.; Wang, F.; Liu, X.; Li, Q.; Tang, Z.; et al. Engineering nanozymes using DNA for catalytic regulation. ACS Appl. Mater. Inter. 2019, 11, 1790–1799.
- Zhang, L.; Qi, Z.; Zou, Y.; Zhang, J.; Xia, W.; Zhang, R.; He, Z.; Cai, X.; Lin, Y.; Duan, S.; et al. Engineering DNA-nanozyme interfaces for rapid detection of dental bacteria. ACS Appl. Mater. Inter. 2019, 11, 30640–30647.
- Wang, Y.; Liu, J.; Adkins, G.B.; Shen, W.; Trinh, M.P.; Duan, L.; Jiang, J.; Zhong, W. Enhancement of the intrinsic peroxidase-like activity of graphitic carbon nitride nanosheets by ssDNAs and its application for detection of exosomes. Anal. Chem. 2017, 89, 12327–12333.
- Zhu, X.; Tang, L.; Wang, J.; Peng, B.; Ouyang, X.; Tan, J.; Yu, J.; Feng, H.; Tang, J. Enhanced peroxidase-like activity of boron nitride quantum dots anchored porous CeO2 nanorods by aptamer for highly sensitive colorimetric detection of kanamycin. Sens. Actuator B Chem. 2021, 330, 129318.
- Tang, Y.; Hu, Y.; Zhou, P.; Wang, C.; Tao, H.; Wu, Y. Colorimetric detection of kanamycin residue in foods based on the aptamer-enhanced peroxidase-mimicking activity of layered WS2 nanosheets. J. Agric. Food Chem. 2021, 69, 2884–2893.
- Hizir, M.S.; Top, M.; Balcioglu, M.; Rana, M.; Robertson, N.M.; Shen, F.; Sheng, J.; Yigit, M.V. Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable peroxidase. Anal. Chem. 2016, 88, 600–605.
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513.
- Zhao, Y.; Li, H.; Lopez, A.; Su, H.; Liu, J. Promotion and inhibition of the oxidase-mimicking activity of nanoceria by phosphate, polyphosphate and DNA. ChemBioChem 2020, 21, 2178–2186.
- Zhang, F.; Wang, S.; Liu, J. Gold nanoparticles adsorb DNA and aptamer probes too strongly, and a comparison with graphene oxide for biosensing. Anal. Chem. 2019, 91, 14743–14750.




