Food safety issues are a worldwide concern. Pathogens, toxins, pesticides, veterinary drugs, heavy metals, and illegal additives are frequently reported to contaminate food and pose a serious threat to human health. Conventional detection methods have difficulties fulfilling the requirements for food development in a modern society. Therefore, novel rapid detection methods are urgently needed for on-site and rapid screening of massive food samples. Due to the extraordinary properties of nanozymes and aptamers, biosensors composed of both of them provide considerable advantages in analytical performances, including sensitivity, specificity, repeatability, and accuracy. They are considered a promising complementary detection method on top of conventional ones for the rapid and accurate detection of food contaminants.
1. Introduction
Food safety has become a major global concern because of its impacts on public health as well as the international food trade. Pathogens, toxins, pesticides, veterinary drugs, heavy metals, and illegal additives are common contaminants in foods [
1]. To ensure food safety, various conventional methods have been developed to determine food contaminants, including traditional plate culture methods, chromatographic methods, enzyme-linked immunosorbent assay (ELISA), and inductively coupled plasma mass spectrometry (ICP-MS). Although these methods demonstrate high accuracy and reliability for detecting food contaminants, they are complicated, time-consuming, and expensive, and they require special equipment and specially trained personnel, which are difficult to meet the requirements for to conduct on-site and rapid screening of massive food samples and are difficult to apply in many places, such as developing countries. Thus, it is urgent to develop rapid, sensitive, low-cost screening methods as complementary ways of ensuring food safety.
Recently, the fast-growing area of nanomaterials has brought a revolution in the field of analysis and detection, especially with the discovery of nanozymes [
2,
3]. Nanozymes usually refer to nanomaterials with enzyme-like activities, including the hydrolase and oxidoreductase families. The hydrolase family of nanozymes consists of nuclease- [
4], esterase- [
5], phosphatase- [
6], and protease-like activity [
7], while oxidoreductase activity includes peroxidase-, oxidase-, catalase-, and superoxide-dismutase-like activity [
8]. Compared with natural enzymes, such as horseradish peroxidase (HRP), nanozymes have several appealing characteristics, such as low cost, high tolerance to harsh conditions (e.g., extreme pH and temperature), facile surface modification, and multifunctionality [
9]. These advantages have attracted increasing research interests over the past decade, in turn elucidating the catalytic mechanism of nanozymes and facilitating the rapid development of the field of nanozymes [
10,
11,
12,
13]. So far, various nanomaterials, including noble-metal- and transition-metal-based nanomaterials [
14,
15,
16], carbon-based nanomaterials [
17,
18], metal–organic framework (MOF)-based nanomaterials [
19] and their hybrids, have been found to possess peroxidase-like or/and oxidase-like activity, which can be utilized to directly detect H
2O
2, glucose, ascorbic acid, and so on [
12,
20,
21,
22]. Achieving the specific detection of food contaminants usually requires the introduction of molecularly imprinted polymers or biorecognition elements, such as antibodies, antimicrobial peptides, bacteriophages, nucleic acid probes, and aptamers [
12,
23,
24,
25]. Among these, aptamers as alternatives to conventional antibodies are one of the most popular biorecognition elements due to their low cost, facile chemical synthesis, flexible chemical modification, and high tolerance to pH and temperature [
26,
27]. Furthermore, specific aptamers for almost all possible targets can be obtained via systematic evolution of ligands by exponential enrichment (SELEX). Up to now, various aptamers were obtained against a wide range of targets, from small molecules such as heavy metals to whole cells such as foodborne pathogens [
28,
29]. More importantly, aptamers’ complementary strands and amplification products are nucleotide sequences, which can reversibly regulate nanozyme activity [
30]. These advantages endow nanozyme-based aptasensors with more diversity and promise for food safety applications, especially novel aptasensors based on the regulation by single-stranded DNA (ssDNA) of nanozyme activity.
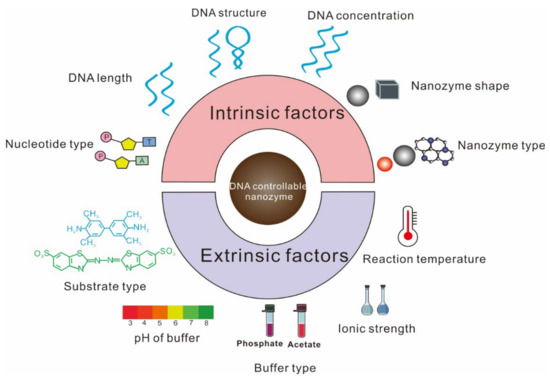
2. Factors Affecting the Regulation by ssDNA of Nanozyme Activity
The influence of ssDNA on nanozyme activity is complicated. Initially, many research works reported that nanozyme activity was inhibited by ssDNA. As research continued, some groups demonstrated that ssDNA actually improved nanozyme activity [
33]. The reasons are yet unclear, but the type of nanozyme and DNA, buffer condition, and temperature in the reaction system were found to be different in these contradicting studies. Hence, to achieve an accurate design of ssDNA-controllable nanozyme biosensors, it is requisite to clearly understand the influence of diverse factors, including intrinsic and extrinsic ones, on the regulation of nanozyme activity by ssDNA (
Figure 1).
Figure 1. Diverse factors that affect the regulation of ssDNA on nanozyme activity.
2.1. Factors Affecting ssDNA Inhibition of Nanozyme Activity
Since nanozyme activity is mainly dependent on their surface properties in the solution, bioconjugation or adsorption of ssDNA on the surface of nanozymes might change the surface properties and affect their activity. In general, adsorbed ssDNA could block the surface active sites or induce the aggregation of nanozymes, thus decreasing the binding sites on nanozymes, resulting in decreased activity [
34,
35]. Thus, the amount of ssDNA aptamer is very important to nanozyme activity. With an increase in aptamer concentration from 2 to 10 μM, the peroxidase-like activity of graphitic carbon nitride@Cu
2O (g-C
3N
4@Cu
2O) nanocomposites gradually reduced and finally reached a plateau [
36], which indicated the saturation of aptamer on the surface of g-C
3N
4@Cu
2O when the amount of aptamer increased to 10 μM. Similar variation trends were observed using Hemin@MOFs nanozyme [
37], gold nanoparticles (AuNPs) nanozyme [
38], and MnCo
2O
4 nanozyme [
39]. Different nucleotide types demonstrated different inhibition ability towards nanozyme activity. The order of inhibiting effects towards MnCo
2O
4 nanozyme activity was: A > G > C > T [
39]. For Mn
3O
4 nanozyme, purine nucleotide (A, G) had more active groups to react with octahedral Mn
3O
4 nanozymes for blocking surface active sites than pyrimidine nucleotide (C, T), demonstrating a stronger inhibitory effect on the oxidase-like activity of Mn
3O
4 [
40]. Theoretically, longer ssDNA would have more binding sites to nanozymes and exhibit a better inhibition effect. In fact, 500 nM of poly A
15 was enough to inhibit the oxidase-like activity of 10 μg/mL MnCo
2O
4. Increasing the number of nucleotides from 15 to 45 did not cause an obvious change in MnCo
2O
4 nanozyme activity, indicating that a saturation of ssDNA was reached on the MnCo
2O
4’s surface [
39].
Wang et al. [
41] found that double-stranded DNA (dsDNA) did not have the ability to inhibit the oxidase-like activity of a mixed-valence state cerium-based metal–organic framework (MVC-MOF). The difference in ability of ssDNA and dsDNA could be ascribed to the selective adsorption of ssDNA by MVC-MOF [
42]. ssDNA has a flexible structure, which contributes to strong π–π stacking interaction between the bases of ssDNA and MOF. Contrarily, dsDNA has a stiff and rigid double-helix structure and cannot wrap around nanomaterials effectively [
37], causing low affinity between dsDNA and nanomaterials. However, when the amount of dsDNA was far more than that of ssDNA, the inhibition effect of the former was comparable to or exceeded that of the latter [
43,
44].
The shape of the nanozyme also affects the interaction of ssDNA and nanozymes. For example, compared with other shapes of Mn
3O
4 nanoparticles (i.e., flower-like, polyhedron, spinel), octahedral Mn
3O
4 nanozymes had more regular surfaces to interact with ssDNA, resulting in a significant inhibition of oxidase-like activity by ssDNA [
40]. Adsorption is the first and key step for realizing DNA regulation of nanozyme activity [
45,
46]. Different nanomaterials have different kinds of adsorption force to ssDNA [
47]. For example, nanoceria adsorbed onto the ssDNA through electrostatic interaction and Lewis acid–base interaction between the phosphate backbone of ssDNA and cerium [
35]. The interaction between DNA and AuNPs was tuned by the combination of various noncovalent forces, including electrostatic interactions, hydrophobic forces, and specific bonds between the chemical groups of DNA bases and gold [
48]. In addition, the main adsorption force between graphene and ssDNA was π–π stacking interaction [
49]. Besides nanozyme type, extrinsic conditions affecting the adsorption forces play an important role in nanozyme activity, including pH and ionic strength. For example, the optimum catalytic condition for MnCo
2O
4 nanozyme was pH 3.0, while the nanozyme activity can be maximally inhibited by ssDNA at pH 4.0 [
39]. Increasing the ionic strength of buffer weakened the inhibition ability of ssDNA toward MnCo
2O
4 nanozyme and enhanced the dissociation ability of ssDNA from the nanozyme’s surface [
39].
2.2. Factors Affecting ssDNA to Improve Nanozyme Activity
With helps from nanotechnology advancement, researchers have found that ssDNA could enhance the catalytic activity of nanomaterials, including noble-metal-based nanomaterials [
33], transition-metal-based nanomaterials, carbon-based nanomaterials [
50], MOFs, and their hybrids. As mentioned above, the adsorption of ssDNA onto the surface of nanomaterials was a prerequisite for ssDNA to regulate the nanozyme activity. For example, ssDNA on the surface of nanozymes could adsorb more substrate (e.g., 3,3′,5,5′-tetramethylbenzidine (TMB)) around the nanozyme due to electrostatic attraction and π–π stacking interaction between TMB and ssDNA, further accelerating electron transfer from TMB to the target sensing molecules (e.g., H
2O
2) and enhancing the nanozyme activity [
45,
51]. Thus, factors affecting both ssDNA–nanomaterial and ssDNA–TMB affinity are important for the enhancement of nanozyme activity by ssDNA. The usual trend of nanozyme activity was to first increase and then decrease with the increase in ssDNA concentration [
52,
53,
54]. However, some data support a different variation trend of nanozyme activity, which is to first increase and then keep stable with the increase of ssDNA concentration [
46,
50,
51,
55,
56]. The reason for the two different trends was unclear. We speculate that the presence of excessive ssDNA may hinder the contact of the nanozyme and TMB in the former trend since it had a wide range of ssDNA concentration. DNA molecules with different structures were also reported to affect the peroxidase-like activity of nanozymes. The order of enhancement of the ability of catalytic activity was: hybridization chain reaction (HCR) products (long dsDNA) > hairpin DNA > ssDNA > dsDNA [
53]. Moreover, different DNA nucleotides had different abilities to enhance the nanozyme activity. The order of enhancement of Fe
3O
4 nanozyme activity was: C > G > T > A [
45]. Cytosine in buffer at pH 4.0 was protonated, which could assist charge neutralization on the surface of Fe
3O
4 nanoparticles and reduce repulsion among DNA, leading to the adsorption of more DNA. The same variation trend was observed by Wang et al. [
50] using protonated graphitic carbon nitride (Pg-C
3N
4) nanosheets as nanozymes. The peroxidase-like activity of 20 bp homo ssDNA (A
20, C
20, G
20, and T
20) modified MoS
2 nanozymes followed the trend of G
20 ≈ T
20 > A
20 > C
20 > no DNA. The explanation from another perspective was given for the weakest activity of C
20-modified MoS
2 nanozymes. Protonated cytosine nucleotides in buffer at pH 4.0 increased the electrostatic repulsion between cytosine and positively charged TMB, resulting in a lower affinity of MoS
2 nanozymes [
51]. Guanine (G) was also reported to significantly enhance the peroxidase-like activity of WS
2 nanosheets [
57], iron-based MOFs modified with acidized carbon nanotubes (MOF/CNTs) [
52], and MIL-53(Fe) [
46]. Purine (A, G) modification demonstrated a remarkable enhancement of the peroxidase-like activity of AuNPs, while pyrimidine (T, C) modification enhanced it slightly, which was attributed to the difference in the interaction between TMB and the surface-adsorbed nucleobases [
58]. In addition, the effect of DNA length on the enhancement of nanozyme activity was also investigated. The longest DNA (ploy C
30) demonstrated the largest enhancement of peroxidase-like activity of Fe
3O
4 nanoparticles due to the presence of more binding sites [
45]. Increasing the number of cytosine nucleotides from 30 to 40, the peroxidase-like activity of Pg-C
3N
4 nanosheets kept steady, which indicated the saturation of ssDNA on the surface of the Pg-C
3N
4 nanosheets [
50]. A slight inhibition of the catalytic activity of MoS
2 nanozymes was found when the number of thymidine nucleotides increased from 30 to 40 [
51].
Besides the intrinsic factors mentioned above, the reaction conditions were also very important for DNA to regulate the nanozyme activity. The buffer pH at 4.0 had been found to be the optimum condition for both the catalytic activity of nanozymes and the inhibition of nanozyme activity by ssDNA [
45,
46,
56,
57]. TMB was the most-used substrate in those literatures. It carries positive charges at pH 4.0 that is below its pKa, which is beneficial to the reaction between ssDNA and TMB [
45]. However, continuously reducing the pH resulted in the protonation of two amino acids of TMB, which made them insusceptible to oxidation [
59]. Moreover, buffer type and concentration were also reported to affect the ability of ssDNA to enhance the peroxidase-like activity of nanozymes. In a phosphate buffer, low-concentration DNA moderately increased the peroxidase-like activity of CeO
2, while DNA in acetate buffer had no effect on the catalytic activity of CeO
2, except for the high-concentration DNA (more than 10 µM), which inhibited the CeO
2 nanozyme activity [
60]. This was because phosphate could compete with ssDNA to bind to CeO
2 nanozymes and enhance their peroxidase-like activity. Low ionic strength increased the peroxidase-like activity of ssDNA-modified WS
2 nanosheets [
57], while high ionic strength could shield the electrostatic reaction, which reduced the interaction among ssDNA, nanozymes, and TMB [
61].
More amazingly, the enhancement or inhibition of the peroxidase-like activity of AuNPs by ssDNA can be controlled by H
2O
2 concentration. In a 10 mM H
2O
2 reaction system, DNA inhibited the peroxidase-like activity of AuNPs in the first 10 min and enhanced the catalytic activity of AuNPs in the next 20 min of the reaction time, while at a lower concentration of H
2O
2 (5 mM), it prolonged the inhibition time to 30 min and enhancement occurred after 30 min [
58]. Moreover, by changing the substrate from the positively charged TMB to the negatively charged 2,2′-azino-bis (3-ethyl benzothiazoline-6-sulfonic acid) (ABTS), the enhanced ability of ssDNA disappeared, and the inhibiting ability of ssDNA appeared [
45,
57].
As previously mentioned, ssDNA can either enhance or inhibit the catalytic activity of nanozymes, and the deciding factor for enhancement or inhibition was to increase or decrease the affinity of substrate (e.g., TMB or ABTS) and nanozymes, respectively, by the introduction of ssDNA. A small change of these factors caused the interaction of ssDNA and nanozymes to be altered, which resulted in variations of the nanozyme activity. Thus, the process of designing aptamer-assisted nanozyme sensing needs to be thoughtful since various factors can affect the regulation of ssDNA on nanozyme activities.
This entry is adapted from the peer-reviewed paper 10.3390/foods11040544