
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduard Roussel | + 1669 word(s) | 1669 | 2022-02-24 09:22:17 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1669 | 2022-03-09 02:05:42 | | |
Video Upload Options
Ultrasound is a widely available, approachable, and relatively inexpensive imaging modality that allows for real-time evaluation of a suspected renal mass without the drawbacks of ionizing radiation and the risk of an MRI. CEUS has several advantages over traditional grayscale ultrasound in the characterization of indeterminate renal masses. It has a distinct value in the characterization of cystic renal masses and has the potential to differentiate benign from malignant renal masses to some extent. Ultrasound molecular imaging could potentially be an extension of the use of CEUS for serial disease monitoring and longitudinal assessment of treatment response, though it remains in preclinical stages of development at this time. While emerging micro-Doppler techniques and elastography have shown some encouraging applications, current evidence is limited, and neither is ready for widespread clinical use.
1. Contrast-Enhanced Ultrasound
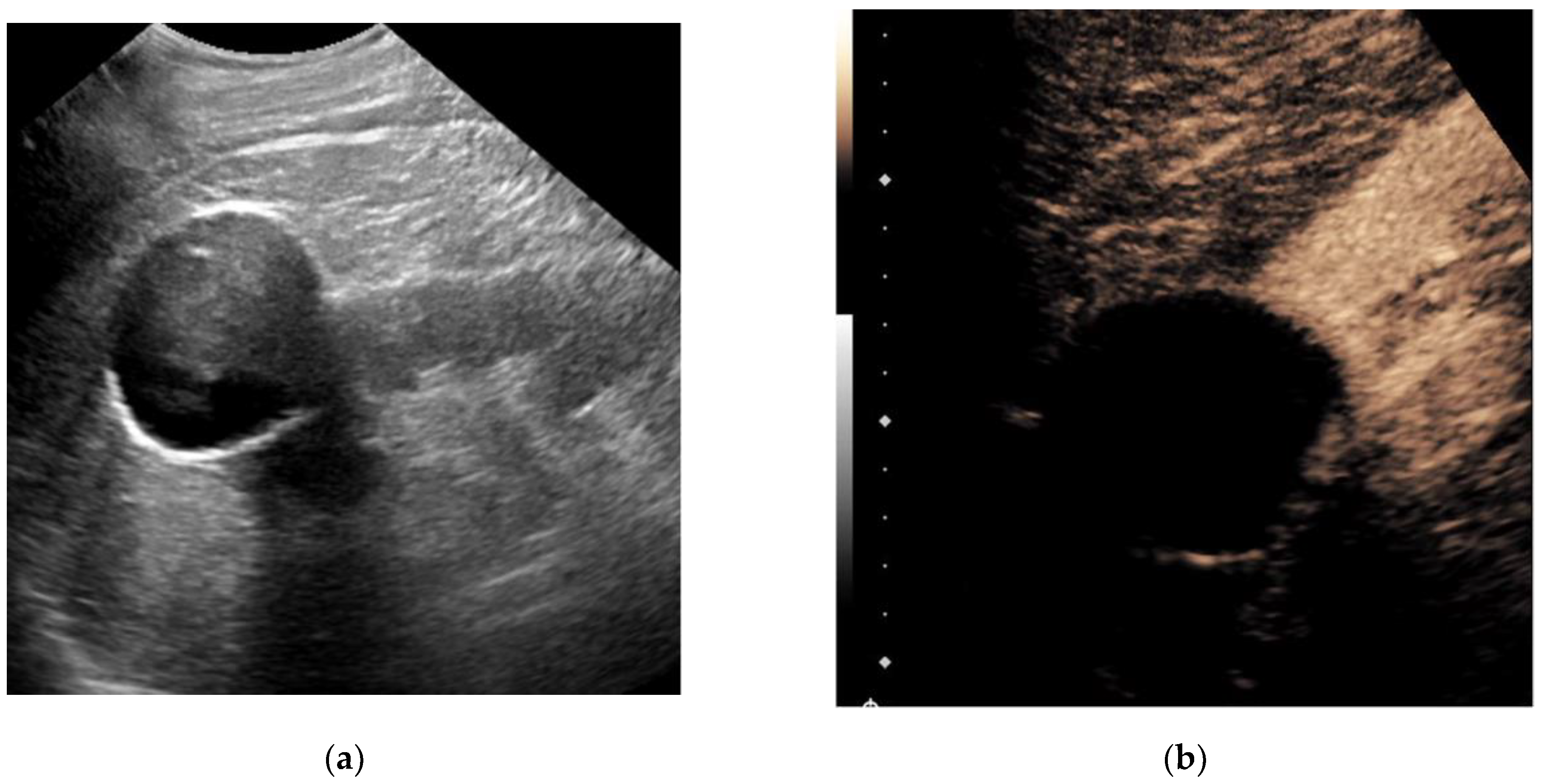
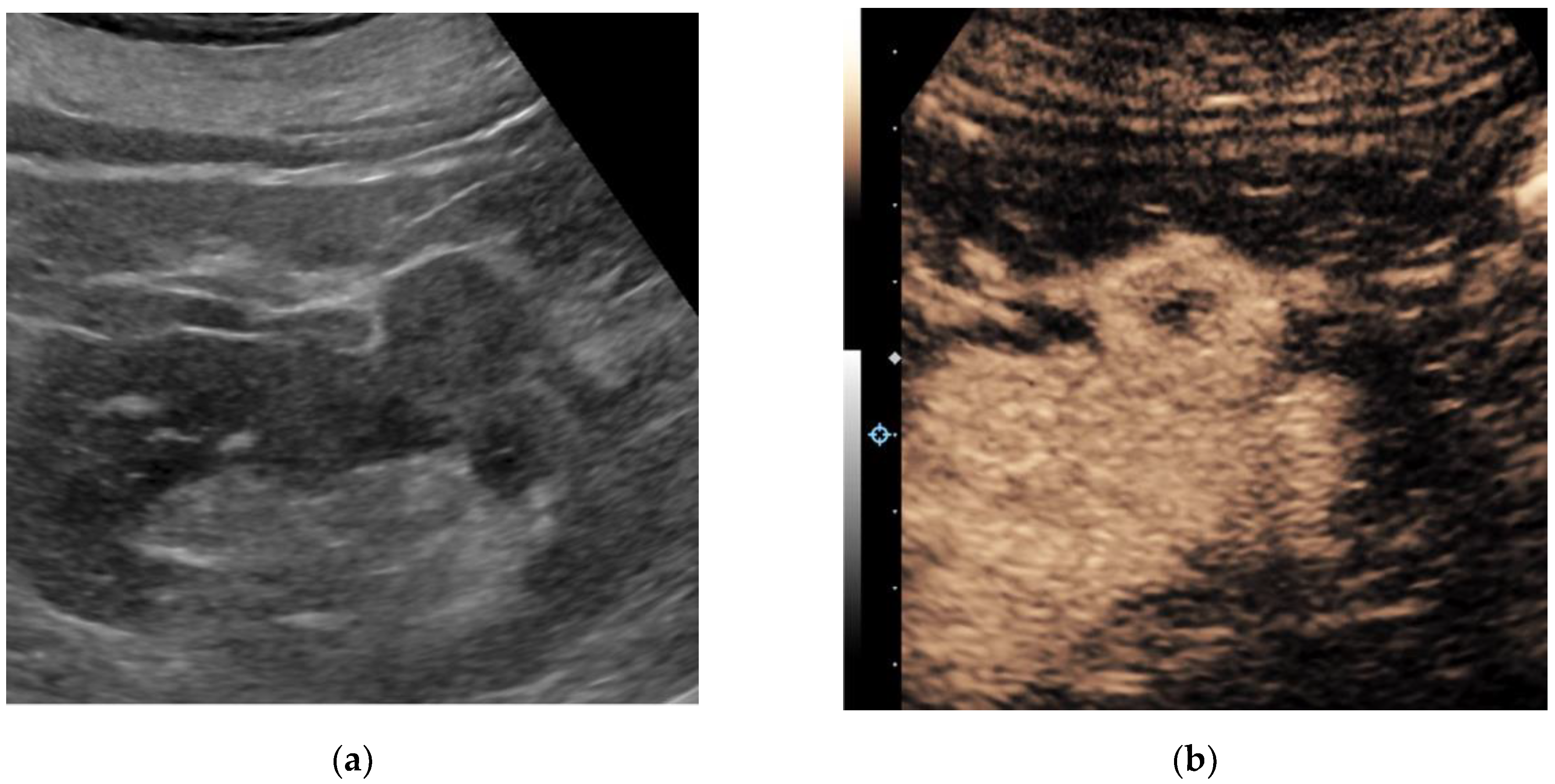
2. Ultrasound Molecular Imaging
3. Elastography
4. Micro-Doppler Techniques
References
- Furrer, M.A.; Spycher, S.C.J.; Büttiker, S.M.; Gross, T.; Bosshard, P.; Thalmann, G.N.; Schneider, M.P.; Roth, B. Comparison of the Diagnostic Performance of Contrast-enhanced Ultrasound with That of Contrast-enhanced Computed Tomography and Contrast-enhanced Magnetic Resonance Imaging in the Evaluation of Renal Masses: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 464–473.
- Fetzer, D.T. Recent Advances in US Vascular Imaging: Highlighting an Important Use Case. Radiology 2020, 298, 91–92.
- Yong, C.; Teo, Y.M.; Kapur, J. Diagnostic performance of contrast-enhanced ultrasound in the evaluation of renal masses in patients with renal impairment. Med. J. Malaysia 2016, 71, 193–198.
- Lu, Q.; Huang, B.J.; Xue, L.Y.; Fan, P.-L.; Wang, W.-P. Differentiation of renal tumor histotypes: Usefulness of quantitative analysis of contrast-enhanced ultrasound. Am. J. Roentgenol. 2015, 205, W335–W342.
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019, 292, 475–488.
- Öztürk, H. Evaluation of Bosniak Type IIF and III Renal Cysts with Contrast-enhanced Ultrasound. J. Belgian. Soc. Radiol. 2016, 100, 12. Available online: http://jbsr.be/articles/10.5334/jbr-btr.843/ (accessed on 23 December 2021).
- Zhou, L.; Tang, L.; Yang, T.; Chen, W. Comparison of contrast-enhanced ultrasound with MRI in the diagnosis of complex cystic renal masses: A meta-analysis. Acta Radiol. 2018, 59, 1254–1263.
- Rübenthaler, J.; Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Clevert, D.A. Evaluation of renal lesions using contrast-enhanced ultrasound (CEUS); a 10-year retrospective European single-centre analysis. Eur. Radiol. 2018, 28, 4542–4549.
- Lerchbaumer, M.H.; Putz, F.J.; Rübenthaler, J.; Rogasch, J.; Jung, E.-M.; Clevert, D.-A.; Hamm, B.; Makowski, M.; Fischer, T. Contrast-enhanced ultrasound (CEUS) of cystic renal lesions in comparison to CT and MRI in a multicenter setting. Clin. Hemorheol. Microcirc. 2020, 75, 419–429.
- Sanz, E.; Hevia, V.; Gómez, V.; Álvarez, S.; Fabuel, J.-J.; Martínez, L.; Rodriguez-Patrón, R.; González-Gordaliza, C.; Burgos, F.-J. Renal Complex Cystic Masses: Usefulness of Contrast-Enhanced Ultrasound (CEUS) in Their Assessment and Its Agreement with Computed Tomography. Curr. Urol. Rep. 2016, 17, 89.
- Ragel, M.; Nedumaran, A.; Makowska-Webb, J. Prospective comparison of use of contrast-enhanced ultrasound and contrast-enhanced computed tomography in the Bosniak classification of complex renal cysts. Ultrasound 2016, 24, 6–16.
- Defortescu, G.; Cornu, J.-N.; Béjar, S.; Giwerc, A.; Gobet, F.; Werquin, C.; Pfister, C.; Nouhaud, F.-X. Diagnostic performance of contrast-enhanced ultrasonography and magnetic resonance imaging for the assessment of complex renal cysts: A prospective study. Int. J. Urol. Off J. Japanese Urol. Assoc. 2017, 24, 184–189.
- Zhou, X.; Yan, F.; Luo, Y.; Peng, Y.-L.; Parajuly, S.S.; Wen, X.-R.; Cai, D.-M.; Li, Y.-Z. Characterization and diagnostic confidence of contrast-enhanced ultrasound for solid renal tumors. Ultrasound Med. Biol. 2011, 37, 845–853.
- Rübenthaler, J.; Paprottka, K.; Marcon, J.; Hameister, E.; Hoffmann, K.; Joiko, N.; Reiser, M.; Clevert, D. Comparison of magnetic resonance imaging (MRI) and contrast-enhanced ultrasound (CEUS) in the evaluation of unclear solid renal lesions. Clin. Hemorheol. Microcirc. 2016, 64, 757–763.
- Wei, S.-P.; Xu, C.-L.; Zhang, Q.; Zhang, Q.-R.; Zhao, Y.-E.; Huang, P.-F.; Xie, Y.-D.; Zhou, C.-S.; Tian, F.-L.; Yang, B. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: Comparison with contrast-enhanced CT. Abdom. Radiol. 2017, 42, 2135–2145.
- Zhang, F.; Li, R.; Li, G.; Jin, L.; Shi, Q.; Du, L. Value of Contrast-Enhanced Ultrasound in the Diagnosis of Renal Cancer and in Comparison With Contrast-Enhanced Computed Tomography: A Meta-analysis. J. Ultrasound. Med. 2019, 38, 903–914.
- Xue, L.-Y.; Lu, Q.; Huang, B.-J.; Li, Z.; Li, C.-X.; Wen, J.-X.; Wang, W.-P. Papillary renal cell carcinoma and clear cell renal cell carcinoma: Differentiation of distinct histological types with contrast-enhanced ultrasonography. Eur. J. Radiol. 2015, 84, 1849–1856.
- Li, C.-X.; Lu, Q.; Huang, B.-J.; Xue, L.-Y.; Yan, L.-X.; Zheng, F.-Y.; Wen, J.-X.; Wang, W.-P. Quantitative evaluation of contrast-enhanced ultrasound for differentiation of renal cell carcinoma subtypes and angiomyolipoma. Eur. J. Radiol. 2016, 85, 795–802.
- Kasoji, S.K.; Chang, E.H.; Mullin, L.B.; Chong, W.K.; Rathmell, W.K.; Dayton, P.A. A Pilot Clinical Study in Characterization of Malignant Renal-cell Carcinoma Subtype with Contrast-enhanced Ultrasound. Ultrason. Imaging 2017, 39, 126–136.
- Lu, Q.; Li, C.; Huang, B.; Xue, L.-Y.; Wang, W.-P. Triphasic and epithelioid minimal fat renal angiomyolipoma and clear cell renal cell carcinoma: Qualitative and quantitative CEUS characteristics and distinguishing features. Abdom. Imaging 2015, 40, 333–342.
- Xue, L.-Y.; Lu, Q.; Huang, B.-J.; Li, C.-X.; Yan, L.-X.; Wang, W.-P. Differentiation of subtypes of renal cell carcinoma with contrast-enhanced ultrasonography. Clin. Hemorheol. Microcirc. 2016, 63, 361–371.
- Schwarze, V.; Marschner, C.; Negrão de Figueiredo, G.; Knösel, T.; Rübenthaler, J.; Clevert, D.-A. Single-center study: The diagnostic performance of contrast-enhanced ultrasound (CEUS) for assessing renal oncocytoma. Scand. J. Urol. 2020, 54, 135–140.
- Ingels, A.; Leguerney, I.; Cournède, P.-H.; Irani, J.; Ferlicot, S.; Sébrié, C.; Benatsou, B.; Jourdain, L.; Pitre-Champagnat, S.; Patard, J.-J.; et al. Ultrasound Molecular Imaging of Renal Cell Carcinoma: VEGFR targeted therapy monitored with VEGFR1 and FSHR targeted microbubbles. Sci. Rep. 2020, 10, 7308.
- Rojas, J.D.; Lin, F.; Chiang, Y.-C.; Chytil, A.; Chong, D.C.; Bautch, V.L.; Rathmell, W.K.; Dayton, P.A. Ultrasound Molecular Imaging of VEGFR-2 in Clear-Cell Renal Cell Carcinoma Tracks Disease Response to Antiangiogenic and Notch-Inhibition Therapy. Theranostics 2018, 8, 141–155. Available online: https://www.thno.org/v08p0141.htm (accessed on 23 December 2021).
- Roussel, E.; Verbiest, A.; Kinget, L.; Boeckx, B.; Zucman-Rossi, J.; Couchy, G.; Caruso, S.; Job, S.; de Reyniès, A.; De Wever, L.; et al. Molecular subtypes and gene expression signatures as prognostic features in fully resected clear cell renal cell carcinoma: A tailored approach to adjuvant trials. Clin. Genitourin. Cancer 2021, 19, e382–e394.
- Roussel, E.; Kinget, L.; Verbiest, A.; Boeckx, B.; Zucman-Rossi, J.; Couchy, G.; Caruso, S.; Baldewijns, M.; Joniau, S.; Van Poppel, H.; et al. Molecular underpinnings of glandular tropism in metastatic clear cell renal cell carcinoma: Therapeutic implications. Acta Oncol. 2021, 60, 1499–1506.
- Onur, M.R.; Poyraz, A.K.; Bozgeyik, Z.; Onur, A.R.; Orhan, I. Utility of semiquantitative strain elastography for differentiation between benign and malignant solid renal masses. J. Ultrasound. Med. 2015, 34, 639–647. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L607593853&from=export (accessed on 23 December 2021).
- Guo, L.-H.; Liu, B.-J.; Xu, H.-X.; Liu, C.; Sun, L.-P.; Zhang, Y.-F.; Xu, J.-M.; Wu, J.; Xu, X.-H. Acoustic radiation force impulse elastography in differentiating renal solid masses: A preliminary experience. Int. J. Clin. Exp. Pathol. 2014, 7, 7469–7476. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L615392918&from=export (accessed on 23 December 2021).
- Keskin, S.; Güven, S.; Keskin, Z.; Özbiner, H.; Kerimoğlu, Ü.; Yeşildağ, A. Strain elastography in the characterization of renal cell carcinoma and angiomyolipoma. Can. Urol. Assoc. J. 2015, 9, e67–e71.
- Inci, M.F.; Kalayci, T.O.; Tan, S.; Karasu, S.; Albayrak, E.; Cakir, V.; Ocal, I.; Ozkan, F. Diagnostic value of strain elastography for differentiation between renal cell carcinoma and transitional cell carcinoma of kidney. Abdom. Radiol. 2016, 41, 1152–1159. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L610856254&from=export (accessed on 23 December 2021).
- Thaiss, W.M.; Bedke, J.; Kruck, S.; Spira, D.; Stenzl, A.; Nikolaou, K.; Horger, M.; Kaufmann, S. Can contrast-enhanced ultrasound and acoustic radiation force impulse imaging characterize CT-indeterminate renal masses? A prospective evaluation with histological confirmation. World J. Urol. 2019, 37, 1339–1346.
- Leong, J.Y.; Wessner, C.E.; Kramer, M.R.; Forsberg, F.; Halpern, E.J.; Lyshchik, A.; Torkzaban, M.; Morris, A.; Byrne, K.; VanMeter, M.; et al. Superb Microvascular Imaging Improves Detection of Vascularity in Indeterminate Renal Masses. J. Ultrasound. Med. Off. J. Am. Inst. Ultrasound. Med. 2020, 39, 1947–1955.
- Mao, Y.; Mu, J.; Zhao, J.; Zhao, L.; Xin, X. The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: A retrospective study. Br. J. Radiol. 2018, 91, 20170601. Available online: https://pubmed.ncbi.nlm.nih.gov/29125337 (accessed on 23 December 2021).




